CASE (CemiplimAb-rwlc Survivorship and Epidemiology): a study in advanced basal cell carcinoma
- PMID: 39797702
- PMCID: PMC11812358
- DOI: 10.1080/14796694.2024.2448416
CASE (CemiplimAb-rwlc Survivorship and Epidemiology): a study in advanced basal cell carcinoma
Abstract
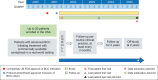
Patients diagnosed with metastatic basal cell carcinoma (BCC) have a poor prognosis. The current standard of care for adults with locally advanced or metastatic BCC who are not candidates for surgery or radiation therapy is treatment with hedgehog pathway inhibitors (HHIs). For patients who progress while on this therapy, further treatment options are limited. There is also a need for real-world clinical practice data on the clinical characteristics, management, disease progression, and survivorship of these patients. The ongoing CemiplimAb-rwlc Survivorship and Epidemiology (CASE) study is a phase IV, multicenter, prospective, noninterventional survivorship and epidemiology cohort study evaluating the effectiveness and safety of cemiplimab, a fully human immunoglobulin G4 monoclonal antibody that blocks the interaction between the programmed cell death-1 (PD-1) receptor and its ligands. This paper describes one cohort of the CASE study of patients with locally advanced or metastatic BCC who have failed or are intolerant of HHIs or for whom HHI therapy is not appropriate. Outcome measures of the study include response to treatment, quality of life, safety, treatment patterns, patient experience, and survival. This study could provide a more complete characterization of this patient population and fill knowledge gaps related to real-world treatment utilization and patient outcomes.Clinical Trial registration: NCT03836105.
Keywords: Basal cell carcinoma; PD-1 inhibitor; cemiplimab; clinical trial; epidemiology; protocol; survivorship.
Conflict of interest statement
Soo J Park reports consulting/advisory roles for Regeneron Pharmaceuticals, Inc. and funding to the institution from Regeneron Pharmaceuticals, Inc. David M Ellison reports stock and other ownership interests in Bristol-Myers Squibb, Illumina, Merck, Pfizer, and Janssen. Ryan Weight reports serving on a speaker’s bureau for Immunocore and Castle Biosciences; consulting/advisory roles for Regeneron Pharmaceuticals, Inc., Pfizer, Castle Biosciences, Immunocore, Novartis, Merck, and the Association of Community Cancer Centers; and steering committee roles for the Association of Community Cancer Centers: ECHO Program. Jade Homsi reports no conflict of interest. Guilherme Rabinowits reports consulting/advisory roles for Replimune, Sanofi, Merck, Exelixis, and Biomedical insights. Emily S Ruiz reports advisory board and consulting fees from Genentech, Feldan Therapeutics, Regeneron Pharmaceuticals, Inc., and Sanofi; and serves on the board of directors for Checkpoint Therapeutics. John Strasswimmer reports consulting/advisory roles for Regeneron Pharmaceuticals, Inc.; serving on a speaker’s bureau for Regeneron Pharmaceuticals, Inc., Sanofi, and Genentech; research funding from Biofrontera, Regeneron Pharmaceuticals, Inc., and Phio Pharmaceuticals, Inc.; and travel, accommodation, or expenses from Regeneron Pharmaceuticals, Inc., and Sanofi. Josh Simmons reports no conflict of interest. Timothy Panella reports no conflict of interest. Ruben GW Quek, and Jean-Francois Pouliot are employees and shareholders of Regeneron Pharmaceuticals, Inc. Nikhil I Khushalani reports grants and advisory board fees from Regeneron Pharmaceuticals, Inc., Bristol-Myers Squibb, Merck, Replimune, and Novartis; advisory board fees from Iovance, Instil Bio, Castle Biosciences, Nektar, Incyte (data safety monitoring committee), AstraZeneca (data safety monitoring committee), and Jounce Therapeutics; grants from GlaxoSmithKline, HUYA, and Celgene; honoraria from Genzyme, an NCCN ORP investigator panel (paid by Pfizer), Nektar (study steering committee), Regeneron Pharmaceuticals, Inc. (study steering committee), BMS (study steering committee), and Replimune (study steering committee); a grant from Modulation Therapeutics; travel support from Regeneron Pharmaceuticals, Inc. and Castle Biosciences; and common stock ownership of Bellicum Pharmaceuticals, Amarin, and Asensus Surgical (formerly Transenetrix). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
-
- Skin Cancer Foundation . Our new approach to a challenging skin cancer statistic. 2021. [cited 2023 Oct 10]. Available from: https://www.skincancer.org/blog/our-new-approach-to-a-challenging-skin-c...
-
- National Cancer Institute . Skin cancer treatment (PDQ®)–health professional version. 2023. [cited 2023 Sep 17]. Available from: https://www.cancer.gov/types/skin/hp/skin-treatment-pdq
-
- Bichakjian C, Armstrong A, Baum C, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78(3):540–559. - PubMed
-
- von Domarus H, Stevens PJ.. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10(6):1043–1060. - PubMed
-
- Bossi P, Ascierto PA, Basset-Seguin N, et al. Long-term strategies for management of advanced basal cell carcinoma with hedgehog inhibitors. Crit Rev Oncol Hematol. 2023;189:104066. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical