Evaporation Kinetics and Final Particle Morphology of Multicomponent Salt Solution Droplets
- PMID: 39797789
- PMCID: PMC11770748
- DOI: 10.1021/acs.jpca.4c07439
Evaporation Kinetics and Final Particle Morphology of Multicomponent Salt Solution Droplets
Abstract
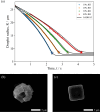
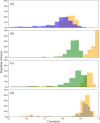
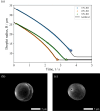
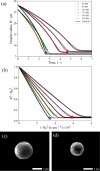
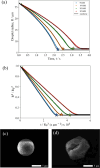
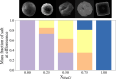
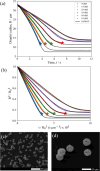
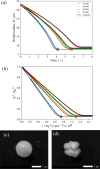
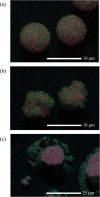
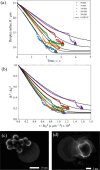
In both nature and industry, aerosol droplets contain complex mixtures of solutes, which in many cases include multiple inorganic components. Understanding the drying kinetics of these droplets and the impact on resultant particle morphology is essential for a variety of applications including improving inhalable drugs, mitigating disease transmission, and developing more accurate climate models. However, the previous literature has only focused on the relationship between drying kinetics and particle morphology for aerosol droplets containing a single nonvolatile component. Here we investigate the drying kinetics of NaCl-(NH4)2SO4, NaCl-NH4NO3, and NaCl-CaCl2 mixed salt aqueous aerosol droplets (25-35 μm radius) and the resulting morphology and composition of the dried microparticles. A comparative kinetics electrodynamic balance was used to measure evaporation profiles for each mixed salt aerosol at a range of relative humidities (RH) (0-50% RH); measurements of the evaporation kinetics are shown to be consistent with predictions from the "Single Aerosol Drying Kinetics and Trajectories" model. Populations of the mixed salt droplets were dried in a falling droplet column under different RH conditions and imaged using scanning electron microscopy to observe the impact of the drying kinetics on the morphology. Energy dispersive spectroscopy was used in tandem to obtain atomic maps and view the impact of drying kinetics on the composition of the resultant particles. It has been shown that the relationship between drying kinetics and dry particle morphology in mixed salt solution droplets is compositionally dependent and determined by the predominant salts that crystallize (i.e., (NH4)2SO4, Na2SO4, or NaCl). The degree of homogeneity in composition throughout the particle microstructure is dependent on the drying rate.
Keywords: aerosol; chloride; crystallization; drying; single-droplet; sulfate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Elwardany A. E.; Badra J.; Sim J.; Khurshid M.; Sarathy M.; Im H. G.. Modeling of Heating and Evaporation of FACE I Gasoline Fuel and its Surrogates; SAE International: 2016. DOI: 10.4271/2016-01-0878. - DOI
-
- Masters K.Spray Drying Handbook; George Godwin Ltd.: 1972.
-
- Gharsallaoui A.; Roudaut G.; Chambin O.; Voilley A.; Saurel R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Research International 2007, 40 (9), 1107–1121. 10.1016/j.foodres.2007.07.004. - DOI
-
- Cotterell M. I.; Willoughby R. E.; Bzdek B. R.; Orr-Ewing A. J.; Reid J. P. A complete parameterisation of the relative humidity and wavelength dependence of the refractive index of hygroscopic inorganic aerosol particles. Atmos. Chem. Phys. 2017, 17 (16), 9837–9851. 10.5194/acp-17-9837-2017. - DOI
LinkOut - more resources
Full Text Sources

