Allocation of Semaglutide According to Coronary Artery Calcium and BMI: Applying the SELECT Trial to MESA
- PMID: 39797878
- PMCID: PMC12273534
- DOI: 10.1016/j.jcmg.2024.10.004
Allocation of Semaglutide According to Coronary Artery Calcium and BMI: Applying the SELECT Trial to MESA
Abstract
Background: Implementation of semaglutide weight loss therapy has been challenging due to drug supply and cost, underscoring a need to identify those who derive the greatest absolute benefit.
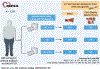
Objectives: Allocation of semaglutide was modeled according to coronary artery calcium (CAC) among individuals without diabetes or established atherosclerotic cardiovascular disease (CVD).
Methods: In this analysis, 3,129 participants in the MESA (Multi-Ethnic Study of Atherosclerosis) without diabetes or clinical CVD met body mass index criteria for semaglutide and underwent CAC scoring on noncontrast cardiac computed tomography. Cox proportional hazards regression assessed the association of CAC with major adverse cardiovascular events (MACE), heart failure (HF), chronic kidney disease (CKD), and all-cause mortality. Risk reduction estimates from the SELECT (Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity) trial (median follow-up: 3.3 years) were applied to observed incidence rates for semaglutide 5-year number-needed-to-treat calculations.
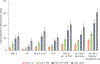
Results: Mean age was 61.2 years, 54% were female, 62% were non-White, mean body mass index was 31.8 kg/m2, and 49% had CAC. Compared with CAC = 0, CAC ≥300 conferred a 2.2-fold higher risk for MACE (HR: 2.16 [95% CI: 1.57-2.99]; P < 0.001). Higher risks for HF (HR: 2.80 [95% CI: 1.81-4.35]; P < 0.001), CKD (HR: 1.59 [95% CI: 1.15-2.22]; P = 0.006), and all-cause mortality (HR: 1.35 [95% CI: 1.08-1.69]; P = 0.009) comparing CAC ≥300 vs CAC = 0 were also observed. There were large 5-year number-needed-to-treat differences between CAC = 0 and CAC ≥300 for MACE (653 vs 79), HF (1,094 vs 144), CKD (1,044 vs 144), and all-cause mortality (408 vs 98).
Conclusions: Measurement of CAC may enhance value of care with weight loss dose semaglutide in those without diabetes or clinical CVD, improving allocation of a limited health care resource.
Keywords: cardiovascular diseases; coronary artery calcium; glucagon-like peptide-1 receptor agonists; obesity; overweight.
Copyright © 2025 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This research was supported by R01HL071739 and R01HL146666 and MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Dr Razavi was supported by the National Heart, Lung, and Blood Institute grant F32HL172499. Dr Razavi was supported by National Heart, Lung, and Blood Institute grants F32HL172499 and L30HL175751. Dr Dzaye has received support from National Institutes of Health grant T32 HL007227. Dr Blaha has received grants from the National Institutes of Health, U.S. Food and Drug Administration, and the American Heart Association; grants and personal fees from Amgen, Bayer, Eli Lilly, and Novo Nordisk; and personal fees from Novartis, Roche, Merck, Boehringer Ingelheim, Vectura, Agepha, and AstraZeneca outside the submitted work. Dr Shapiro has received grants (to institution) from PCORI, DCRI, Amgen, Boehringer Ingelheim, 89Bio, Esperion, Genentech, Novartis, Ionis, Merck, and New Amsterdam; Scientific Advisory Boards with Amgen, Agepha, Ionis, Novartis, Precision BioScience, Novo Nordisk, and New Amsterdam; and has received fees as a consultant with Ionis, Novartis, Regeneron, Aidoc, Shanghai Pharma Biotherapeutics, and Kaneka. Dr Al-Mallah has received grants from Siemens; and consultation fees from Jubulant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Cecchini M, Vuik S. The heavy burden of obesity. In: OECD. The Heavy Burden of Obesity: The Economics of Prevention. OECD Publishing, 2019:16–39. 10.1787/3c6ec454-en - DOI
Publication types
MeSH terms
Substances
Grants and funding
- N01 HC095168/HL/NHLBI NIH HHS/United States
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- T32 HL007227/HL/NHLBI NIH HHS/United States
- R01 HL146666/HL/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- L30 HL175751/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- R01 HL071739/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- F32 HL172499/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

