Immunogenicity of yellow fever vaccine co-administered with 13-valent pneumococcal conjugate vaccine in rural Gambia: A cluster-randomised trial
- PMID: 39798436
- PMCID: PMC11797555
- DOI: 10.1016/j.vaccine.2025.126712
Immunogenicity of yellow fever vaccine co-administered with 13-valent pneumococcal conjugate vaccine in rural Gambia: A cluster-randomised trial
Abstract
Introduction: Because booster doses of pneumococcal conjugate vaccine (PCV) may be given at a similar time to yellow fever vaccine (YF), it is important to assess the immune response to YF when co-administered with PCV. This has been investigated during a reduced-dose PCV trial in The Gambia.
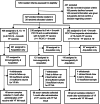
Methods: In this phase 4, parallel-group, cluster-randomized trial, healthy infants aged 0-10 weeks were randomly allocated to receive either a two-dose schedule of PCV13 with a booster dose co-administered with YF vaccine at age 9 months (1 + 1 co-administration) or YF vaccine administered separately at age 10 months (1 + 1 separate) or the standard three early doses of PCV13 with YF vaccine at age 9 months (3 + 0 separate). Blood samples were collected 28-35 days post-vaccination and YF neutralizing antibody (NA) titres were measured. Proportions with seroprotective YF NA titres ≥ 1:8 were calculated with 95 % confidence intervals (CI). Non-inferiority was demonstrated if the lower limit of the CI for the difference in proportions between the co-administration and separate groups was greater than - 10 %.
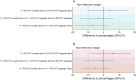
Results: Forty-eight, 66, and 98 participants enrolled in 3 + 0 separate, 1 + 1 co-administration, and 1 + 1 separate groups respectively had NA results. Per protocol analysis of the 3 + 0 separate, 1 + 1 co-administration, 1 + 1 separate, and the combined 1 + 1 separate and 3 + 0 separate groups found that 81 %, 85 %, 92 %, and 88 % of participants respectively had YF NA titres ≥1:8. Results were similar with analysis by intention-to-treat. The difference in proportions comparing 1 + 1 co-administration and 1 + 1 separate groups was -7 % (95 % CI, -18 % to 3 %). The difference between 1 + 1 co-administration and 3 + 0 separate groups was 4 % (95 % CI, -10 % to 15 %). There was no statistical difference in the YF seroresponse when the YF vaccine was co-administered with PCV or administered separately.
Conclusions: No evidence was found of the non-inferiority of the seroresponse to YF vaccine when co-administered with PCV13. The levels of YF NA attaining seroprotection (NT ≥1:8) were high in all groups. PCV13 co-administered with YF vaccine at 9 months does not affect seroresponse to YF vaccine. http://www.isrctn.org/ - ISRCTN72821613.
Keywords: Co-administration; Neutralizing antibody titre; Pneumococcal conjugate vaccine; The Gambia; Yellow fever vaccine.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Grant Mackenzie reports financial support was provided by Bill & Melinda Gates Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- World Health Organization . Disease Outbreak News; Yellow fever in African Region (AFRO) 2024, March. https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON510 Available at.
-
- Organization WH Yellow fever fact sheet:(revised in December 2009) Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire. 2010;85(05):33–36. - PubMed
-
- Maryanne E., Jay S. Principles and practice of pediatric infectious diseases. 4th ed. Elsevier Inc.; United States: 2012. Understanding, controlling, and preventing infectious diseases; pp. 76–83.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

