Mice with reduced protease-activated receptor 4 reactivity show decreased venous thrombosis and platelet procoagulant activity
- PMID: 39798922
- PMCID: PMC11992619
- DOI: 10.1016/j.jtha.2024.12.031
Mice with reduced protease-activated receptor 4 reactivity show decreased venous thrombosis and platelet procoagulant activity
Abstract
Background: Hypercoagulation and thrombin generation are major risk factors for venous thrombosis. Sustained thrombin signaling through protease-activated receptor (PAR) 4 promotes platelet activation, phosphatidylserine exposure, and subsequent thrombin generation. A single nucleotide polymorphism in PAR4 (rs2227376) changes proline to leucine extracellular loop 3, which decreases PAR4 reactivity and is associated with a lower risk for venous thromboembolism (VTE) in a genome wide association studies meta-analysis.
Objectives: The goal of this study was to determine the mechanism for the association of rs2227376 with a reduced risk of VTE using mice with a homologous mutation (PAR4-P322L).
Methods: Venous thrombosis was examined using our recently generated PAR4-P322L mice in the inferior vena cava stasis and stenosis models. Coagulation and clot stability were measured using rotational thromboelastometry. Thrombin-generating potential was measured in platelet-rich plasma. Phosphatidylserine surface expression and platelet-neutrophil aggregates were analyzed using flow cytometry.
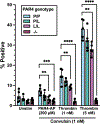
Results: Mice heterozygous (PAR4P/L) or homozygous (PAR4L/L) at position 310 had reduced sizes of venous clots at 48 hours. PAR4P/L and PAR4L/L platelets had progressively decreased phosphatidylserine in response to thrombin and convulxin, in addition to decreased thrombin generation and decreased PAR4-mediated platelet-neutrophil aggregation.
Conclusion: The leucine allele in extracellular loop 3, PAR4-322L, leads to fewer procoagulant platelets, decreased endogenous thrombin potential, and reduced platelet-neutrophil aggregation. This decreased ability to generate thrombin and bind to neutrophils offers a mechanism for PAR4's role in VTE, highlighting a key role for PAR4 signaling.
Keywords: animal model; blood platelets; protease-activated receptor 4; single nucleotide polymorphisms; thrombin receptor.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interests There are no competing interests to disclose.
Figures






Update of
-
Mice with Reduced PAR4 Reactivity show Decreased Venous Thrombosis and Platelet Procoagulant Activity.bioRxiv [Preprint]. 2024 Oct 17:2024.10.14.617127. doi: 10.1101/2024.10.14.617127. bioRxiv. 2024. Update in: J Thromb Haemost. 2025 Apr;23(4):1278-1288. doi: 10.1016/j.jtha.2024.12.031. PMID: 39463946 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

