Advances of Hydroxyapatite Nanoparticles in Dental Implant Applications
- PMID: 39799064
- PMCID: PMC12142779
- DOI: 10.1016/j.identj.2024.11.020
Advances of Hydroxyapatite Nanoparticles in Dental Implant Applications
Abstract
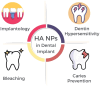
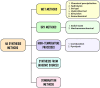
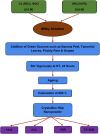
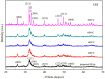
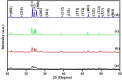
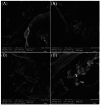
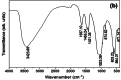
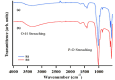
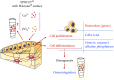
Hydroxyapatite nanoparticles (HANPs) are becoming increasingly crucial in dental implant applications as they are highly compatible with biological systems, actively support biological processes, and closely resemble bone minerals. This review covers the latest progress in how HANPs are made, studied, and used in dentistry. It looks at critical methods for creating HANPs, such as sol-gel, microwave hydrothermal synthesis, and biomimetic approaches, and how they affect the particles' size, structure, and activity. The green synthesis method illustrated a new door to synthesize HAp for maintaining biocompatibilityand increasing antibacterial properties. The review also explores how HANPs improve the integration of implants with bone, support bone growth, and help treat sensitive teeth based on various laboratory and clinical studies. The usage of HAp in dentin and enamel shows higher potentiality through FTIR, XPS, XRD, EDS, etc., for mechanical stability and biological balance compared to natural teeth. Additionally, the use of HANPs in dental products like toothpaste and mouthwash is discussed, highlighting its potential to help rebuild tooth enamel and fight bacteria. There are some challenges for long-term usage against oral bacteria, but doping with inorganic materials, like Zn, has already solved this periodontal problem. Much more research is still essential to estimate the fabrication variation based on patient problems and characteristics. Still, it has favorable outcomes regarding its bioactive nature and antimicrobial properties. Due to their compatibility with biological tissues and ability to support bone growth, HANPs hold great promise for advancing dental materials and implant technology, potentially leading to better dental care and patient outcomes.
Keywords: Biodegradation; Bone regeneration; Dental treatments; Hydroxyapatite; Implant.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures












Similar articles
-
Impact of hydroxyapatite nanoparticles on the cellular processes of stem cells derived from dental tissue sources.Cell Tissue Res. 2025 Jun;400(3):319-330. doi: 10.1007/s00441-025-03962-6. Epub 2025 Mar 18. Cell Tissue Res. 2025. PMID: 40100346
-
Interaction of hydroxyapatite nanoparticles with endothelial cells: internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway.Int J Nanomedicine. 2017 Aug 10;12:5781-5795. doi: 10.2147/IJN.S140179. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28848353 Free PMC article.
-
Biocompatibility and periodontal regenerative potential of hydroxyapatite nanoparticles from Portunus Sanguinolentus Shells: A crystallographic, morphological, and molecular gene expression analysis.J Dent. 2025 Jun;157:105762. doi: 10.1016/j.jdent.2025.105762. Epub 2025 Apr 15. J Dent. 2025. PMID: 40246056
-
Nanoscale hydroxyapatite particles for bone tissue engineering.Acta Biomater. 2011 Jul;7(7):2769-81. doi: 10.1016/j.actbio.2011.03.019. Epub 2011 Apr 1. Acta Biomater. 2011. PMID: 21440094 Review.
-
Hydroxyapatite: A journey from biomaterials to advanced functional materials.Adv Colloid Interface Sci. 2023 Nov;321:103013. doi: 10.1016/j.cis.2023.103013. Epub 2023 Oct 7. Adv Colloid Interface Sci. 2023. PMID: 37839281 Review.
Cited by
-
Nano-Hydroxyapatite-Based Mouthwash for Comprehensive Oral Care: Activity Against Bacterial and Fungal Pathogens with Antioxidant and Anti-Inflammatory Action.Materials (Basel). 2025 Jul 30;18(15):3567. doi: 10.3390/ma18153567. Materials (Basel). 2025. PMID: 40805446 Free PMC article.
-
A review on innovations in hydroxyapatite: advancing sustainable and multifunctional dental implants.Odontology. 2025 Apr 10. doi: 10.1007/s10266-025-01096-3. Online ahead of print. Odontology. 2025. PMID: 40208376 Review.
References
-
- Mohanraj V.J., Chen Y. Nanoparticles - A review. Trop J Pharma Res. 2007;5(1) doi: 10.4314/tjpr.v5i1.14634. - DOI
-
- Banfield J.F., Zhang H. Nanoparticles in the environment. Rev Mineral Geochem. 2001;44(1):1–58. doi: 10.2138/rmg.2001.44.01. - DOI
-
- Hartland, A., Lead, J. R., Slaveykova, V., O'Carroll, D., & Valsami-Jones, E. (2013). The environmental significance of natural nanoparticles. https://archive-ouverte.unige.ch/unige:29558
-
- Pasero M., Kampf A.R., Ferraris C., Pekov I.V., Rakovan J., White T.J. Nomenclature of the apatite supergroup minerals. Eur J Mineral. 2010;22(2):163–179. doi: 10.1127/0935-1221/2010/0022-2022. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

