Inhibited peroxidase activity of peroxiredoxin 1 by palmitic acid exacerbates nonalcoholic steatohepatitis in male mice
- PMID: 39799115
- PMCID: PMC11724923
- DOI: 10.1038/s41467-025-55939-2
Inhibited peroxidase activity of peroxiredoxin 1 by palmitic acid exacerbates nonalcoholic steatohepatitis in male mice
Abstract
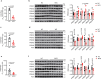
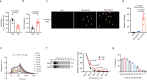
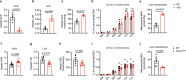
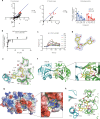
Reactive oxygen species exacerbate nonalcoholic steatohepatitis (NASH) by oxidizing macromolecules; yet how they promote NASH remains poorly understood. Here, we show that peroxidase activity of global hepatic peroxiredoxin (PRDX) is significantly decreased in NASH, and palmitic acid (PA) binds to PRDX1 and inhibits its peroxidase activity. Using three genetic models, we demonstrate that hepatic PRDX1 protects against NASH in male mice. Mechanistically, PRDX1 suppresses STAT signaling and protects mitochondrial function by scavenging hydrogen peroxide, and mitigating the oxidation of protein tyrosine phosphatases and lipid peroxidation. We further identify rosmarinic acid (RA) as a potent agonist of PRDX1. As revealed by the complex crystal structure, RA binds to PRDX1 and stabilizes its peroxidatic cysteine. RA alleviates NASH through specifically activating PRDX1's peroxidase activity. Thus, beyond revealing the molecular mechanism underlying PA promoting oxidative stress and NASH, our study suggests that boosting PRDX1's peroxidase activity is a promising intervention for treating NASH.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

