A membrane-targeted photoswitch restores physiological ON/OFF responses to light in the degenerate retina
- PMID: 39799138
- PMCID: PMC11724966
- DOI: 10.1038/s41467-025-55882-2
A membrane-targeted photoswitch restores physiological ON/OFF responses to light in the degenerate retina
Abstract
The lack of effective therapies for visual restoration in Retinitis pigmentosa and macular degeneration has led to the development of new strategies, such as optogenetics and retinal prostheses. However, visual restoration is poor due to the massive light-evoked activation of retinal neurons, regardless of the segregation of visual information in ON and OFF channels, which is essential for contrast sensitivity and spatial resolution. Here, we show that Ziapin2, a membrane photoswitch that modulates neuronal capacitance and excitability in a light-dependent manner, is capable of reinstating, in mouse and rat genetic models of photoreceptor degeneration, brisk and sluggish ON, OFF, and ON-OFF responses in retinal ganglion cells evoked by full-field stimuli, with reactivation of their excitatory and inhibitory conductances. Intravitreally injected Ziapin2 in fully blind rd10 mice restores light-driven behavior and optomotor reflexes. The results indicate that Ziapin2 is a promising molecule for reinstating physiological visual responses in the late stages of retinal degeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.B., V.S., G.L., and F.B. are among the inventors of the patent application “Photochromic compounds” (PCT/IB2019/054530). The remaining authors declare no competing interests. Inclusion and Ethics: The Authors followed the recommendations of the “Global Code of Conduct” when designing, executing, and reporting their research.
Figures
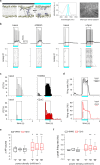
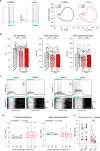


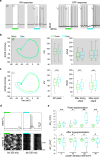



References
MeSH terms
Grants and funding
- GMR22T2013/Fondazione Telethon (Telethon Foundation)
- GR-2021-12374630/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- 861423/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 Marie Skłodowska-Curie Actions (H2020 Excellent Science - Marie Skłodowska-Curie Actions)
- 2020XBFEMS/Ministero dell'Istruzione, dell'Università e della Ricerca (Ministry of Education, University and Research)
LinkOut - more resources
Full Text Sources
Miscellaneous

