Targeting KRAS: from metabolic regulation to cancer treatment
- PMID: 39799325
- PMCID: PMC11724471
- DOI: 10.1186/s12943-024-02216-3
Targeting KRAS: from metabolic regulation to cancer treatment
Abstract
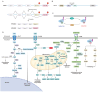
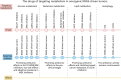
The Kirsten rat sarcoma viral oncogene homolog (KRAS) protein plays a key pathogenic role in oncogenesis, cancer progression, and metastasis. Numerous studies have explored the role of metabolic alterations in KRAS-driven cancers, providing a scientific rationale for targeting metabolism in cancer treatment. The development of KRAS-specific inhibitors has also garnered considerable attention, partly due to the challenge of acquired treatment resistance. Here, we review the metabolic reprogramming of glucose, glutamine, and lipids regulated by oncogenic KRAS, with an emphasis on recent insights into the relationship between changes in metabolic mechanisms driven by KRAS mutant and related advances in targeted therapy. We also focus on advances in KRAS inhibitor discovery and related treatment strategies in colorectal, pancreatic, and non-small cell lung cancer, including current clinical trials. Therefore, this review provides an overview of the current understanding of metabolic mechanisms associated with KRAS mutation and related therapeutic strategies, aiming to facilitate the understanding of current challenges in KRAS-driven cancer and to support the investigation of therapeutic strategies.
Keywords: Cancer metabolism; KRAS inhibitors; KRAS-driven cancers; Metabolic reprogramming.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Liu J, Kang R, Tang D. The KRAS-G12C inhibitor: activity and resistance. Cancer Gene Ther. 2022;29(7):875–8. 10.1038/s41417-023-00692-1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

