Personalising glioblastoma medicine: explant organoid applications, challenges and future perspectives
- PMID: 39799339
- PMCID: PMC11724554
- DOI: 10.1186/s40478-025-01928-x
Personalising glioblastoma medicine: explant organoid applications, challenges and future perspectives
Abstract
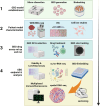
Glioblastoma (GBM) is a highly aggressive adult brain cancer, characterised by poor prognosis and a dismal five-year survival rate. Despite significant knowledge gains in tumour biology, meaningful advances in patient survival remain elusive. The field of neuro-oncology faces many disease obstacles, one being the paucity of faithful models to advance preclinical research and guide personalised medicine approaches. Recent technological developments have permitted the maintenance, expansion and cryopreservation of GBM explant organoid (GBO) tissue. GBOs represent a translational leap forward and are currently the state-of-the-art in 3D in vitro culture system, retaining brain cancer heterogeneity, and transiently maintaining the immune infiltrate and tumour microenvironment (TME). Here, we provide a review of existing brain cancer organoid technologies, in vivo xenograft approaches, evaluate in-detail the key advantages and limitations of this rapidly emerging technology, and consider solutions to overcome these difficulties. GBOs currently hold significant promise, with the potential to emerge as the key translational tool to synergise and enhance next-generation omics efforts and guide personalised medicine approaches for brain cancer patients into the future.
Keywords: Brain cancer; Explants; Glioblastoma; Heterogeneity; Organoids; Patient-derived; Personalised medicine.
© 2025. Crown.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have approved the manuscript. Competing interests: The authors declare no competing interests.
Figures


References
-
- Leighton J (1951) A sponge matrix method for tissue culture; formation of organized aggregates of cells in vitro. J Natl Cancer Inst 12:545–561 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

