Biomechanics in the tumor microenvironment: from biological functions to potential clinical applications
- PMID: 39799341
- PMCID: PMC11724500
- DOI: 10.1186/s40164-024-00591-7
Biomechanics in the tumor microenvironment: from biological functions to potential clinical applications
Abstract
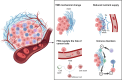
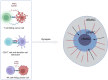
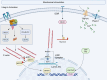
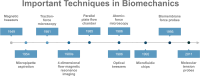
Immune checkpoint therapies have spearheaded drug innovation over the last decade, propelling cancer treatments toward a new era of precision therapies. Nonetheless, the challenges of low response rates and prevalent drug resistance underscore the imperative for a deeper understanding of the tumor microenvironment (TME) and the pursuit of novel targets. Recent findings have revealed the profound impacts of biomechanical forces within the tumor microenvironment on immune surveillance and tumor progression in both murine models and clinical settings. Furthermore, the pharmacological or genetic manipulation of mechanical checkpoints, such as PIEZO1, DDR1, YAP/TAZ, and TRPV4, has shown remarkable potential in immune activation and eradication of tumors. In this review, we delved into the underlying biomechanical mechanisms and the resulting intricate biological meaning in the TME, focusing mainly on the extracellular matrix, the stiffness of cancer cells, and immune synapses. We also summarized the methodologies employed for biomechanical research and the potential clinical translation derived from current evidence. This comprehensive review of biomechanics will enhance the understanding of the functional role of biomechanical forces and provide basic knowledge for the discovery of novel therapeutic targets.
Keywords: Biomechanical target; Biomechanics; Extracellular matrix; Stiffness; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Ma T, Chen Z, Chai Y, Gongye X, Xia P, Qu C, et al. Research progress on immunotherapy targeting the tumor immune microenvironment for cholangiocarcinoma. Oncol Transl Med. 2023;9(2):49–55. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

