Epigenetic regulation and post-translational modifications of ferroptosis-related factors in cardiovascular diseases
- PMID: 39799367
- PMCID: PMC11724467
- DOI: 10.1186/s13148-024-01809-5
Epigenetic regulation and post-translational modifications of ferroptosis-related factors in cardiovascular diseases
Abstract
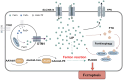
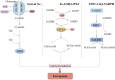
As an important element of the human body, iron participates in numerous physiological and biochemical reactions. In the past decade, ferroptosis (a form of iron-dependent regulated cell death) has been reported to contribute to the pathogenesis and progression of various diseases. The stability of iron in cardiomyocytes is crucial for the maintenance of normal physiological cardiac activity. Ferroptosis has been detected in many cardiovascular diseases (CVDs), including coronary heart disease, myocardial ischemia-reperfusion injury, heart failure, and chemotherapy-induced myocardial damage. In cardiomyocytes, epigenetic regulation and post-translational modifications regulate the expression of ferroptosis-related factors, maintain iron homeostasis, and participate in the progression of CVDs. Currently, there is no detailed mechanism to explain the relationship between epigenetic regulation and ferroptosis in CVDs. In this review, we provide an initial summary of the core mechanisms of ferroptosis in cardiomyocytes, with first focus on the epigenetic regulation and expression of ferroptosis-related factors in the context of common cardiovascular diseases. We anticipate that the new insights into the pathogenesis of CVDs provided here will inspire the development of clinical interventions to specifically target the active sites of these factors, reducing the harmfulness of ferroptosis to human health.
Keywords: Cardiovascular diseases; Epigenetic regulation; Ferroptosis; Post-translation modification.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Xiao FJ, et al. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem Biophys Res Commun. 2019;515(3):448–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2023-MS-053/Natural Science Foundation of Liaoning Province
- 2023-MS-051/Natural Science Foundation of Liaoning Province
- 2022-MS-078/Natural Science Foundation of Liaoning Province
- 82371982/the National Natural Science Foundation of China
- RC220223/Shenyang Middle younger Scientific and Technological Innovation Support Plan
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

