CMPK2 promotes NLRP3 inflammasome activation via mtDNA-STING pathway in house dust mite-induced allergic rhinitis
- PMID: 39799434
- PMCID: PMC11726638
- DOI: 10.1002/ctm2.70180
CMPK2 promotes NLRP3 inflammasome activation via mtDNA-STING pathway in house dust mite-induced allergic rhinitis
Abstract
Background: House dust mite (HDM) is the leading allergen for allergic rhinitis (AR). Although allergic sensitisation by inhaled allergens renders susceptible individuals prone to developing AR, the molecular mechanisms driving this process remain incompletely elucidated.
Objective: This study aimed to elucidate the molecular mechanisms underlying HDM-induced AR.
Methods: We examined the expression of cytidine/uridine monophosphate kinase 2 (CMPK2), STING and the NLRP3 inflammasome in both AR patients and mice. Additionally, we investigated the role of CMPK2 and STING in the activation of the NLRP3 inflammasome in AR.
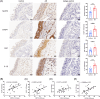
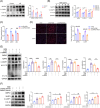
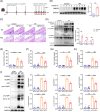
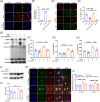
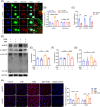
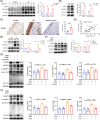
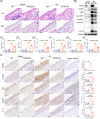
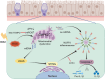
Results: The expression of CMPK2, STING and the NLRP3 inflammasome was significantly increased in the nasal mucosa of AR patients compared to non-AR controls. A positive correlation was found between CMPK2 expression and the levels of STING, NLRP3, ASC, CASP1 and IL-1β. HDM treatment up-regulated the expression of CMPK2, and CMPK2 overexpression enhanced NLRP3 inflammasome activation in human nasal epithelial cells (HNEPCs). Additionally, mitochondrial reactive oxygen species (mtROS) production following HDM exposure contributed to mitochondrial dysfunction and the release of mitochondrial DNA (mtDNA), which activated the cyclic GMP-AMP synthase (cGAS)-STING pathway. Remarkably, depletion of mtDNA or inhibition of STING signalling reduced HDM-induced NLRP3 inflammasome activation in HNEPCs. In vivo, genetic knockout of CMPK2 or STING alleviated NLRP3 inflammasome activation and ameliorated clinical symptoms of AR in mice.
Conclusions: Our results suggest that HDM promotes the activation of NLRP3 inflammasome through the up-regulation of CMPK2 and ensuing mtDNA-STING signalling pathway, hence revealing additional therapeutic target for AR.
Key points: Cytidine/uridine monophosphate kinase 2 (CMPK2) expression is up-regulated in the nasal mucosa of patients and mice with allergic rhinitis (AR). CMPK2 caused NLRP3 inflammasome activation via mitochondrial DNA (mtDNA)-STING pathway. Blocking CMPK2 or STING signalling significantly reduced the activation of NLRP3 in house dust mite (HDM)-challenged mice and human nasal epithelial cells (HNEPCs).
Keywords: CMPK2; NLRP3 inflammasome; STING; allergic rhinitis; mitochondrial DNA.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Wise SK, Damask C, Roland LT, et al. International consensus statement on allergy and rhinology: allergic rhinitis – 2023. Int Forum Allergy Rhinol. 2023;13(4):293‐859. - PubMed
-
- Bernstein JA, Bernstein JS, Makol R, Ward S. Allergic rhinitis: a review. JAMA. 2024;331(10):866‐877. - PubMed
-
- Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Prim. 2020;6(1):95. - PubMed
-
- Zuberbier T, Lötvall J, Simoens S, Subramanian SV, Church MK. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69(10):1275‐1279. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
