Kinetically-derived maximal dose (KMD) confirms lack of human relevance for high-dose effects of octamethylcyclotetrasiloxane (D4)
- PMID: 39799522
- PMCID: PMC11774993
- DOI: 10.1007/s00204-024-03914-z
Kinetically-derived maximal dose (KMD) confirms lack of human relevance for high-dose effects of octamethylcyclotetrasiloxane (D4)
Abstract
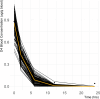
The kinetically-derived maximal dose (KMD) is defined as the maximum external dose at which kinetics are unchanged relative to lower doses, e.g., doses at which kinetic processes are not saturated. Toxicity produced at doses above the KMD can be qualitatively different from toxicity produced at lower doses. Here, we test the hypothesis that high-dose-dependent toxicological effects of octamethylcyclotetrasiloxane (D4) occur secondary to kinetic overload. Octamethylcyclotetrasiloxane (D4) is a volatile, highly lipophilic monomer used to produce silicone polymers, which are ingredients in many consumer products and used widely in industrial applications and processes. Chronic inhalation at D4 concentrations 104 times greater than human exposures produces mild effects in rat respiratory tract, liver weight increase and pigment accumulation, nephropathy, uterine endometrial epithelial hyperplasia, non-significant increased uterine endometrial adenomas, and reduced fertility secondary to inhibition of rat-specific luteinizing hormone (LH) surge. Mechanistic studies indicate a lack of human relevance for most of these effects. Respiratory tract effects arise in rats due to direct epithelial contact with mixed vapor/aerosols and increased liver weight is a rodent-specific adaptative induction of drug-metabolizing hepatic enzymes. D4 is not mutagenic or genotoxic, does not interact with dopamine receptors, and interacts at ERα with potency insufficient to cause uterine effects or to alter the LH surge in rats. These mechanistic findings suggest high-dose-dependence of the toxicological effects secondary to kinetic overload, a hypothesis that can be tested when appropriate kinetic data are available that can be probed for the existence of a KMD. We applied Bayesian analysis with differential equations to information from kinetic studies on D4 to build statistical distributions of plausible values of the Km and Vmax for D4 elimination. From those distributions of likely Km and Vmax values, a set of Michaelis-Menten equations were generated that are likely to represent the slope function for the relationship between D4 exposure and blood concentration. The resulting Michaelis-Menten functions were then investigated using a change-point methodology known as the "kneedle" algorithm to identify the probable KMD range. We validated our Km and Vmax using out of sample data. Analysis of the Michaelis-Menten elimination curve generated from those Vmax and Km values indicates a KMD with an interquartile range of 230.0-488.0 ppm [2790-5920 mg/m3; 9.41-19.96 µM]. The KMD determined here for D4 is consistent with prior work indicating saturation of D4 metabolism at approximately 300 ppm [3640 mg/m3; 12.27 µM] and supports the hypothesis that many adverse effects of D4 arise secondary to high-dose-dependent events, likely due to mechanisms of action that cannot occur at concentrations below the KMD. Regulatory methods to evaluate D4 for human health protection should avoid endpoint data from rodents exposed to D4 above the KMD range and future toxicological testing should focus on doses below the KMD range.
Keywords: Human relevance; Kinetically-derived maximum dose (KMD); Maximum tolerated dose (MTD); Pharmacokinetics/toxicokinetics; Regulatory policy; Threshold.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: This work was funded by the Silicones Environmental Health & Safety Center (SEHSC) to conduct this KMD evaluation. SEHSC reviewed and commented on the initial draft of the manuscript. The methodologies, analyses, interpretations, content of the manuscript, and the decision to submit it for publication were made solely by the authors and did not depend on approval from SEHSC.
Figures


Similar articles
-
Octamethylcyclotetrasiloxane (D4) lacks endocrine disruptive potential via estrogen pathways.Arch Toxicol. 2025 Apr;99(4):1431-1443. doi: 10.1007/s00204-024-03896-y. Epub 2025 Feb 20. Arch Toxicol. 2025. PMID: 39976757 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
Cited by
-
Octamethylcyclotetrasiloxane (D4) lacks endocrine disruptive potential via estrogen pathways.Arch Toxicol. 2025 Apr;99(4):1431-1443. doi: 10.1007/s00204-024-03896-y. Epub 2025 Feb 20. Arch Toxicol. 2025. PMID: 39976757 Free PMC article.
References
-
- Andersen ME (1981) Saturable metabolism and its relationship to toxicity. Crit Rev Toxicol 9:105–150 - PubMed
-
- Andersen ME (2022) Assessing modes of action, measures of tissue dose and human relevance of rodent toxicity endpoints with octamethylcyclotetrasiloxane (D4). Toxicol Lett 357:57–72. 10.1016/j.toxlet.2021.12.020 - PubMed
-
- Andersen ME, Sarangapani R, Reitz RH, Gallavan RH, Dobrev ID, Plotzke KP (2001) Physiological modeling reveals novel pharmacokinetic behavior for inhaled octamethylcyclotetrasiloxane in rats. Toxicol Sci 60(2):214–231 - PubMed
-
- Baker S (2010) Potential for octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane to interact with and activate the dopamine D2 receptor in rat striatal membranes. Silicones Environmental, Health and Safety Council Report
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

