Time to weight plateau with tirzepatide treatment in the SURMOUNT-1 and SURMOUNT-4 clinical trials
- PMID: 39800653
- PMCID: PMC12096058
- DOI: 10.1111/cob.12734
Time to weight plateau with tirzepatide treatment in the SURMOUNT-1 and SURMOUNT-4 clinical trials
Abstract
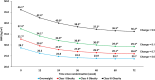
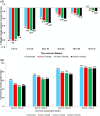
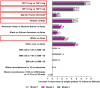
The rate of weight reduction during obesity treatment declines over time and eventually reaches a weight plateau. We investigated factors associated with time to weight plateau (TTWP) in tirzepatide-treated participants with obesity or overweight in a post-hoc analysis of SURMOUNT-1 and SURMOUNT-4 trials. Participants adherent to tirzepatide treatment and achieving ≥5% weight loss by primary endpoint (week 72 SURMOUNT-1; week 88 SURMOUNT-4) were included. Weight plateau was defined as a weight change <5% over a 12-week interval and all subsequent 12-week intervals. TTWP was time from randomization to the start of the first 12-week interval. Association between baseline characteristics and TTWP was assessed. Overall, 1438 participants in SURMOUNT-1 and 259 in SURMOUNT-4 were included. Across BMI categories (overweight, class I, II, and III), median TTWP in SURMOUNT-1 was 24.3, 26.0, 36.1, and 36.1 weeks, respectively (p <.05, class II and III vs. overweight). By week 72, 90.2%, 88.9%, 87.6%, and 87.8% of participants in SURMOUNT-1 had reached a weight plateau across respective BMI categories [Correction added on 22 January 2025, after first online publication: The "72%" has been changed to "72" in this version.]. Higher doses of tirzepatide (10/15 mg), younger age, and female sex were more likely to reach a weight plateau later. Results in SURMOUNT-4 were similar. In this post-hoc analysis, most participants reached a weight plateau by week 72. Higher doses of tirzepatide, younger age, and female sex were associated with a longer TTWP. Further research into modifiers of weight reduction phases with tirzepatide may inform treatment decisions for its use in chronic weight management. Clinical Trial Registration: ClinicalTrials.gov, identifiers NCT04184622 (SURMOUNT-1) and NCT04660643 (SURMOUNT-4), available at http://www.clinicaltrials.gov/.
Keywords: obesity; tirzepatide; weight plateau.
© 2025 The Author(s). Clinical Obesity published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Conflict of interest statement
Deborah Horn participated on advisory boards for Amgen, Eli Lilly and Company, and Novo Nordisk and received consulting fees from Amgen, Eli Lilly and Company, Gelesis, and Novo Nordisk. She served as an unpaid member of the World Obesity Clinical Care Committee, The Obesity Society Annual Program Committee, and The Obesity Society Clinical Science Section Committee. Scott Kahan participated on advisory boards and received consulting fees from Boehringer, Carmot, Currax, Eli Lilly and Company, Novo Nordisk, Pfizer, and Vivus. He served on the board of directors for The Obesity Society and Obesity Action Coalition and had roles in committee leadership for the American Diabetes Association and the Endocrine Society. Rachel Batterham participated on advisory boards, received honoraria, and/or received consulting fees from Eli Lilly and Company, Epitomee Medical Ltd., Gila Therapeutics Ltd., International Medical Press, Medscape, Novo Nordisk, and ViiV Healthcare. She served in unpaid leadership positions including the Royal College of Physicians (RCP) special advisor on obesity; Chair of RCP Advisory Group on nutrition, weight and health; Member of RCP Advisory Group on health inequalities; Council member of British Obesity and Metabolic Surgery Society; Member of National Bariatric Surgery Registry; Trustee for the Association for the Study of Obesity; Co‐chair of NHS England clinical advisory group on specialist weight management; Obesity Health Alliance Strategy Group; Co‐chair of International Federation for the Surgery for Obesity and Metabolic Diseases European Chapter; Committee member of NICE Weight Management Advisory Group; Chair and founding member of Obesity Empowerment Network UK; and Clinical Committee member of the European Society for Endocrinology. Sylvia Gonsahn‐Bollie received consulting fees and was a grant recipient from the Black Physicians Healthcare Network. She participated on an advisory board for Novo Nordisk. She received travel fees and speaker honoraria from the Obesity Medicine Association. She served in unpaid leadership roles for the Obesity Medicine Association, National Wellness Institute, and Black Physicians Healthcare Network. Rachel Batterham, Dachuang Cao, Clare Lee, Madhumita Murphy, Sylvia Gonsahn‐Bollie, Farai Chigutsa, Adam Stefanski, and Julia P Dunn are employees and shareholders of Eli Lilly and Company.
Figures

References
-
- EMA . Summary of Product Characteristics, Mounjaro. https://www.ema.europa.eu/en/documents/product-information/mounjaro-epar...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

