Moderate-intensity interval exercise exacerbates cardiac lipotoxicity in high-fat, high-calories diet-fed mice
- PMID: 39800728
- PMCID: PMC11725574
- DOI: 10.1038/s41467-025-55917-8
Moderate-intensity interval exercise exacerbates cardiac lipotoxicity in high-fat, high-calories diet-fed mice
Abstract
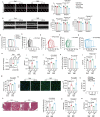
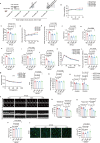
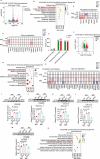
Physical exercise is a cornerstone for preventing diet-induced obesity, while it is unclear whether physical exercise could offset high-fat, high-calories diet (HFCD)-induced cardiac dysfunction. Here, mice were fed with HFCD and simultaneously subjected to physical exercise. As expected, physical exercise prevented HFCD-induced whole-body fat deposition. However, physical exercise exacerbated HFCD-induced cardiac damage. Further metabolomic analysis results showed that physical exercise induced circulating lipid redistribution, leading to excessive cardiac lipid uptake and lipotoxicity. Our study provides valuable insights into the cardiac effects of exercise in mice fed with HFCD, suggesting that counteracting the negative effect of HFCD by simultaneous physical exercise might be detrimental. Moreover, inappropriate physical exercise may damage certain organs even though it leads to weight loss and overall metabolic benefits. Of note, the current findings are based on animal experiments, the generalizability of these findings beyond this specific diet and mouse strain remains to be further explored.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Hales C. M., Carroll M. D., Fryar C. D. & Ogden C. L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 360, 1–8 (2020). - PubMed
-
- Valenzuela P. L., et al. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 20, 475–494 (2023). - PubMed
-
- Brody, H. Fatty liver disease. Nature551, S85 (2017). - PubMed
-
- Costantino, S. et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur. Heart J.40, 997–1008 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

