Human parietal epithelial cells as Trojan horses in albumin overload
- PMID: 39800742
- PMCID: PMC11725586
- DOI: 10.1038/s41598-024-84972-2
Human parietal epithelial cells as Trojan horses in albumin overload
Abstract
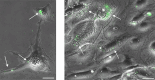
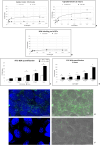
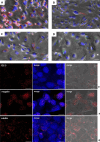
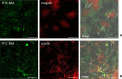
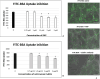
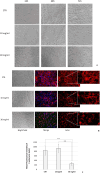
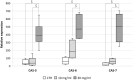
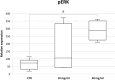
Parietal Epithelial Cells (PECs) activation and proliferation are common to several distinct forms of glomerulopathies. Due to several stimuli, PECs can change to a progenitor (CD24+ and CD133/2+) or a pro-sclerotic (CD44+) phenotype. In addition, PECs, which are constantly exposed to filtered albumin, are known to be involved in albumin internalization, but how this mechanism occurs is unknown. We hypothesized that PECs can transport albumin via receptor-mediated endocytosis and that albumin overload may affect the state of PECs. Conditionally immortalized human PECs (hPECs) were incubated with different albumin concentrations at different times. Albumin internalization studies were performed. Protein expression was assessed using In-Cell Western and immunofluorescence. Cell morphology was analyzed by phase-contrast microscopy and F-actin staining. We demonstrate that hPECs internalize albumin via receptor-mediated mechanisms. Under albumin stimulation, megalin, cubilin, ClC-5, CD133/2, CD24, and CD44 were upregulated. The increase of pERK1/2, the upregulation of ROCK1, ROCK2, caspase -3, -6, and -7, and the morphological changes associated with loss of F-actin fibers indicated that inflammation, proliferation and apoptosis mechanisms had been activated. Our results demonstrate that long-term exposure to high doses of albumin induces up-regulation of molecules involved in the tubular protein uptake machinery and suggest that albumin overload is able to trigger a regenerative process as well as an activation state which might lead in vivo to glomerular crescent formation.
Keywords: Albumin; CD44; ClC-5; Cubilin; Megalin; hPECs.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Kuppe, C. et al. Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney Int.88, 990–998 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

