The interconnective role of the UPS and autophagy in the quality control of cancer mitochondria
- PMID: 39800773
- PMCID: PMC11725563
- DOI: 10.1007/s00018-024-05556-x
The interconnective role of the UPS and autophagy in the quality control of cancer mitochondria
Abstract
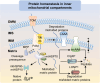
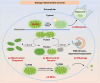
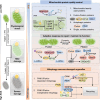
Uncontrollable cancer cell growth is characterized by the maintenance of cellular homeostasis through the continuous accumulation of misfolded proteins and damaged organelles. This review delineates the roles of two complementary and synergistic degradation systems, the ubiquitin-proteasome system (UPS) and the autophagy-lysosome system, in the degradation of misfolded proteins and damaged organelles for intracellular recycling. We emphasize the interconnected decision-making processes of degradation systems in maintaining cellular homeostasis, such as the biophysical state of substrates, receptor oligomerization potentials (e.g., p62), and compartmentalization capacities (e.g., membrane structures). Mitochondria, the cellular hubs for respiration and metabolism, are implicated in tumorigenesis. In the subsequent sections, we thoroughly examine the mechanisms of mitochondrial quality control (MQC) in preserving mitochondrial homeostasis in human cells. Notably, we explored the relationships between mitochondrial dynamics (fusion and fission) and various MQC processes-including the UPS, mitochondrial proteases, and mitophagy-in the context of mitochondrial repair and degradation pathways. Finally, we assessed the potential of targeting MQC (including UPS, mitochondrial molecular chaperones, mitochondrial proteases, mitochondrial dynamics, mitophagy and mitochondrial biogenesis) as cancer therapeutic strategies. Understanding the mechanisms underlying mitochondrial homeostasis may offer novel insights for future cancer therapies.
Keywords: Autophagy-lysosome; Cancer therapy; Mitochondrial chaperones; Mitochondrial proteases; Mitophagy; Protein quality control; UPS.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no competing interests. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable.
Figures



References
-
- Buchberger A, Bukau B, Sommer T (2010) Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell 40(2):238–252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

