Critical Role for Transglutaminase 2 in Scleroderma Skin Fibrosis and in the Development of Dermal Sclerosis in a Mouse Model of Scleroderma
- PMID: 39800950
- PMCID: PMC12209749
- DOI: 10.1002/art.43104
Critical Role for Transglutaminase 2 in Scleroderma Skin Fibrosis and in the Development of Dermal Sclerosis in a Mouse Model of Scleroderma
Abstract
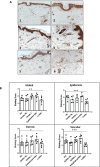
Objective: Scleroderma is a life-threatening autoimmune disease characterized by inflammation, tissue remodeling, and fibrosis. This study aimed to investigate the expression and function of transglutaminase 2 (TGM2) in scleroderma skin and experimentally induced dermal fibrosis to determine its potential role and therapeutic implications.
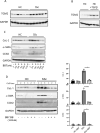
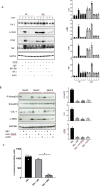
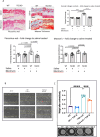
Methods: We performed immunohistochemistry on skin sections to assess TGM2 expression and localization, and protein biochemistry of both systemic sclerosis-derived and healthy control dermal fibroblasts to assess TGM2 expression, function, and extracellular matrix deposition in the presence of TGM2 inhibiting and transforming growth factor (TGF)-β neutralizing antibodies and a small-molecule inhibitor of the TGF-βRI kinase. Mice with a complete deficiency of TGM2 (Tgm2-/-) were investigated in the bleomycin-induced model of skin fibrosis.
Results: TGM2 was found to be widely expressed in both control and scleroderma skin samples, as well as in cultured fibroblasts. Scleroderma fibroblasts exhibited elevated TGM2 expression, which correlated with increased expression of fibrosis markers (Col-1, αSMA, and CCN2). Inhibition of TGM2 using an inhibiting antibody reduced the expression of key markers of fibrosis. The effects of TGM2 inhibition were similar to those observed with TGF-β neutralization, suggesting a potential crosstalk between TGM2 and TGF-β signaling. Moreover, TGM2 knockout mice showed significantly reduced dermal fibrosis compared with wild type mice. In vitro experiments with TGM2-deleted fibroblasts demonstrated impaired cell migration and collagen matrix contraction, which could be partially restored by exogenous TGF-β.
Conclusion: TGM2 can regulate several key profibrotic activities of TGF-β suggesting that attenuating TGM2 function may be of benefit in severe forms of connective tissue disease with skin fibrosis.
© 2025 UCB and The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

Similar articles
-
EphB2 Receptor Promotes Dermal Fibrosis in Systemic Sclerosis.Arthritis Rheumatol. 2024 Aug;76(8):1303-1316. doi: 10.1002/art.42858. Epub 2024 May 15. Arthritis Rheumatol. 2024. PMID: 38589317 Free PMC article.
-
[Influence and mechanism of extracellular vesicles derived from human dermal papilla cells on skin fibrosis in mice].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025 Jun 20;41(6):559-568. doi: 10.3760/cma.j.cn501225-20240925-00348. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2025. PMID: 40588404 Free PMC article. Chinese.
-
The stromal vascular fraction mitigates bleomycin-induced skin fibrosis in mice by modulating vascular lesions and secreting antifibrotic factors at different stages of disease.Stem Cell Res Ther. 2025 Jul 1;16(1):328. doi: 10.1186/s13287-025-04470-8. Stem Cell Res Ther. 2025. PMID: 40597400 Free PMC article.
-
Cyclophosphamide for connective tissue disease-associated interstitial lung disease.Cochrane Database Syst Rev. 2018 Jan 3;1(1):CD010908. doi: 10.1002/14651858.CD010908.pub2. Cochrane Database Syst Rev. 2018. PMID: 29297205 Free PMC article.
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
References
-
- Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390(10103):1685–1699. - PubMed
-
- Allanore Y, Simms R, Distler O, et al. Systemic sclerosis. Nat Rev Dis Primers 2015;23:15002. - PubMed
-
- Volkmann ER, Varga J. Emerging targets of disease‐modifying therapy for systemic sclerosis. Nat Rev Rheumatol 2019;15:208–224. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

