Purification and characterization of a thermophilic NAD+-dependent lactate dehydrogenase from Moorella thermoacetica
- PMID: 39801223
- PMCID: PMC12051018
- DOI: 10.1002/2211-5463.13964
Purification and characterization of a thermophilic NAD+-dependent lactate dehydrogenase from Moorella thermoacetica
Abstract
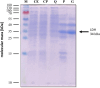
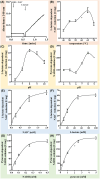
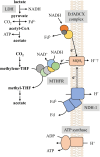
Oxidation of lactate under anaerobic dark fermentative conditions poses an energetic problem. The redox potential of the lactate/pyruvate couple is too electropositive to reduce the physiological electron carriers NAD(P)+ or ferredoxin. However, the thermophilic, anaerobic, and acetogenic model organism Moorella thermoacetica can grow on lactate but was suggested to have a NAD+-dependent lactate dehydrogenase (LDH), based on enzyme assays in cell-free extract. LDHs of thermophilic and anaerobic bacteria are barely characterized but have a huge biotechnological potential. Here, we have purified the LDH from M. thermoacetica by classical chromatography. Lactate-dependent NAD+ reduction was observed with high rates. Electron bifurcation was not observed. At pH 8 and 65 °C, the LDH had a specific activity of 60 U·mg-1 for lactate oxidation, but NADH-driven pyruvate reduction was around four times faster with an activity of 237 U·mg-1. Since lactate formation is preferred by the enzyme, further modifications of the LDH can be suggested to improve the kinetics of this enzyme making it a promising candidate for biotechnological applications.
Keywords: acetogen; anaerobe; lactate; purification; thermophile.
© 2025 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Lactate metabolism in strictly anaerobic microorganisms with a soluble NAD+ -dependent l-lactate dehydrogenase.Environ Microbiol. 2021 Aug;23(8):4661-4672. doi: 10.1111/1462-2920.15657. Epub 2021 Jul 16. Environ Microbiol. 2021. PMID: 34190373
-
Roles of d-Lactate Dehydrogenases in the Anaerobic Growth of Shewanella oneidensis MR-1 on Sugars.Appl Environ Microbiol. 2019 Jan 23;85(3):e02668-18. doi: 10.1128/AEM.02668-18. Print 2019 Feb 1. Appl Environ Microbiol. 2019. PMID: 30504209 Free PMC article.
-
A novel hexameric NADP+ -reducing [FeFe] hydrogenase from Moorella thermoacetica.FEBS J. 2024 Feb;291(3):596-608. doi: 10.1111/febs.16989. Epub 2023 Nov 7. FEBS J. 2024. PMID: 37885325
-
The redox switch/redox coupling hypothesis.Neurochem Int. 2006 May-Jun;48(6-7):523-30. doi: 10.1016/j.neuint.2005.12.036. Epub 2006 Mar 10. Neurochem Int. 2006. PMID: 16530294 Review.
-
Flavin-Based Electron Bifurcation, Ferredoxin, Flavodoxin, and Anaerobic Respiration With Protons (Ech) or NAD+ (Rnf) as Electron Acceptors: A Historical Review.Front Microbiol. 2018 Mar 14;9:401. doi: 10.3389/fmicb.2018.00401. eCollection 2018. Front Microbiol. 2018. PMID: 29593673 Free PMC article. Review.
References
-
- Drake HL, Gößner AS and Daniel SL (2008) Old acetogens, new light. Ann N Y Acad Sci 1125, 100–128. - PubMed
-
- Katsyv A, Essig M, Bedendi G, Sahin S, Milton RD and Müller V (2023) Characterization of ferredoxins from the thermophilic, acetogenic bacterium Thermoanaerobacter kivui . FEBS J 290, 4107–4125. - PubMed
-
- Ljungdahl LG (1986) The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol 40, 415–450. - PubMed
-
- Weghoff MC, Bertsch J and Müller V (2015) A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ Microbiol 17, 670–677. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

