The discovery of a new nonbile acid modulator of Takeda G protein-coupled receptor 5: An integrated computational approach
- PMID: 39801251
- PMCID: PMC11726147
- DOI: 10.1002/ardp.202400423
The discovery of a new nonbile acid modulator of Takeda G protein-coupled receptor 5: An integrated computational approach
Abstract
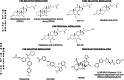
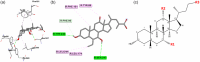
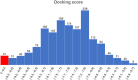
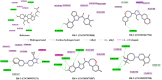
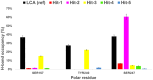
The Takeda G protein-coupled receptor 5 (TGR5), also known as GPBAR1 (G protein-coupled bile acid receptor), is a membrane-type bile acid receptor that regulates blood glucose levels and energy expenditure. These essential functions make TGR5 a promising target for the treatment of type 2 diabetes and metabolic disorders. Currently, most research on developing TGR5 agonists focuses on modifying the structure of bile acids, which are the endogenous ligands of TGR5. However, TGR5 agonists with nonsteroidal structures have not been widely explored. This study aimed at discovering new TGR5 agonists using bile acid derivatives as a basis for a computational approach. We applied a combination of pharmacophore-based, molecular docking, and molecular dynamic (MD) simulation to identify potential compounds as new TGR5 agonists. Through pharmacophore screening and molecular docking, we identified 41 candidate compounds. From these, five candidates were selected based on criteria including pharmacophore features, a docking score of less than 9.2 kcal/mol, and similarity in essential interaction patterns with a reference ligand. Biological assays of the five hits confirmed that Hit-3 activates TGR5 similarly to the bile acid control. This was supported by MD simulation results, which indicated that a hydrogen bond interaction with Tyr240 is involved in TGR5 activation. Hit-3 (CSC089939231) represents a new nonsteroidal lead that can be further optimized to design potent TGR5 agonists.
Keywords: INT‐777; TGR5; molecular docking; nonbile acid; pharmacophore.
© 2025 The Author(s). Archiv der Pharmazie published by Wiley‐VCH GmbH on behalf of Deutsche Pharmazeutische Gesellschaft.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Identification of potential dual agonists of FXR and TGR5 using e-pharmacophore based virtual screening.Mol Biosyst. 2015 May;11(5):1305-18. doi: 10.1039/c5mb00137d. Mol Biosyst. 2015. PMID: 25787676
-
Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies.J Med Chem. 2008 Mar 27;51(6):1831-41. doi: 10.1021/jm7015864. Epub 2008 Feb 29. J Med Chem. 2008. PMID: 18307294
-
Discovery and characterization of small-molecule TGR5 ligands with agonistic activity.Eur J Med Chem. 2024 Oct 5;276:116616. doi: 10.1016/j.ejmech.2024.116616. Epub 2024 Jun 28. Eur J Med Chem. 2024. PMID: 38996653
-
Update on the development of TGR5 agonists for human diseases.Eur J Med Chem. 2024 May 5;271:116462. doi: 10.1016/j.ejmech.2024.116462. Epub 2024 Apr 28. Eur J Med Chem. 2024. PMID: 38691888 Review.
-
Steroidal scaffolds as FXR and GPBAR1 ligands: from chemistry to therapeutical application.Future Med Chem. 2015;7(9):1109-35. doi: 10.4155/fmc.15.54. Future Med Chem. 2015. PMID: 26132522 Review.
References
-
- Di Leva F. S., Di Marino D., Limongelli V., Handb. Exp. Pharmacol. 2019, 256, 111. - PubMed
-
- Sepe V., Distrutti E., Limongelli V., Fiorucci S., Zampella A., Future Med. Chem. 2015, 7(9), 1109. - PubMed
-
- Keitel V., Stindt J., Häussinger D., Handb. Exp. Pharmacol. 2019, 256, 19. - PubMed
-
- Sindhu T., Srinivasan P., J. Recept. Signal Transduct. 2017, 37(2), 109. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

