C3/C3aR Bridges Spinal Astrocyte-Microglia Crosstalk and Accelerates Neuroinflammation in Morphine-Tolerant Rats
- PMID: 39801259
- PMCID: PMC11725764
- DOI: 10.1111/cns.70216
C3/C3aR Bridges Spinal Astrocyte-Microglia Crosstalk and Accelerates Neuroinflammation in Morphine-Tolerant Rats
Abstract
Aims: Communication within glial cells acts as a pivotal intermediary factor in modulating neuroimmune pathology. Meanwhile, an increasing awareness has emerged regarding the detrimental role of glial cells and neuroinflammation in morphine tolerance (MT). This study investigated the influence of crosstalk between astrocyte and microglia on the evolution of morphine tolerance.
Methods: Sprague-Dawley rats were intrathecally treated with morphine twice daily for 9 days to establish morphine-tolerant rat model. Tail-flick latency test was performed to identify the analgesic effect of morphine. The role of microglia, astrocyte and C3-C3aR axis in morphine tolerance were elucidated by real-time quantitative polymerase chain reaction, Western blot, and immunofluorescence.
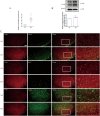
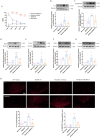
Results: Chronic morphine treatment notably promoted the activation of microglia, upregulated the production of proinflammatory mediators (interleukin-1 alpha (IL-1α), tumor necrosis factor alpha (TNFα), and complement component 1q (C1q)). Simultaneously, it programed astrocytes to a pro-inflammatory phenotype (A1), which mainly expresses complement 3 (C3) and serping1. PLX3397 (a colony-stimulating factor 1 receptor (CSF1R) inhibitor), Compstain (a C3 inhibitor) and SB290157(a C3aR antagonist) could reverse the above pathological process and alleviate morphine tolerance to different extents.
Conclusion: Our findings identify C3-C3aR axis as an amplifier of microglia-astrocyte crosstalk, neuroinflammation and a node for therapeutic intervention in morphine tolerance.
Keywords: A1 astrocyte; C3/C3aR; microglia‐astrocyte crosstalk; morphine tolerance.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
All the authors have approved the manuscript and agree with submission to Experimental Neurology. The authors declare no conflicts of interest.
Figures
Similar articles
-
Predicting Neuroinflammation in Morphine Tolerance for Tolerance Therapy from Immunostaining Images of Rat Spinal Cord.PLoS One. 2015 Oct 5;10(10):e0139806. doi: 10.1371/journal.pone.0139806. eCollection 2015. PLoS One. 2015. PMID: 26437460 Free PMC article.
-
Salidroside Attenuates Epilepsy and Cognitive Dysfunction in Rats by Downregulating Complement C3-C3aR Pathway-Mediated Activation of Microglia and Astrocytes.Neurochem Res. 2025 Aug 21;50(5):271. doi: 10.1007/s11064-025-04514-8. Neurochem Res. 2025. PMID: 40839226
-
A Novel H2S Donor Alleviates Neuroinflammation and Seizures by Inhibiting the C3-C3aR Pathway.J Neurosci Res. 2025 May;103(5):e70041. doi: 10.1002/jnr.70041. J Neurosci Res. 2025. PMID: 40317781
-
Modulation of microglia can attenuate neuropathic pain symptoms and enhance morphine effectiveness.Pharmacol Rep. 2008 May-Jun;60(3):297-307. Pharmacol Rep. 2008. PMID: 18622054 Review.
-
Linking depression and neuroinflammation: Crosstalk between glial cells.Eur J Pharmacol. 2025 May 15;995:177408. doi: 10.1016/j.ejphar.2025.177408. Epub 2025 Feb 19. Eur J Pharmacol. 2025. PMID: 39984011 Review.
Cited by
-
Microglial TAK1 promotes neurotoxic astrocytes and cognitive impairment in LPS-induced hippocampal neuroinflammation.J Biol Chem. 2025 Jun;301(6):110225. doi: 10.1016/j.jbc.2025.110225. Epub 2025 May 9. J Biol Chem. 2025. PMID: 40349778 Free PMC article.
-
Complement C1q is associated with neuroinflammation and mediates the association between amyloid-β and tau pathology in Alzheimer's disease.Transl Psychiatry. 2025 Jul 17;15(1):247. doi: 10.1038/s41398-025-03458-5. Transl Psychiatry. 2025. PMID: 40675947 Free PMC article.
References
-
- Szabo I., Chen X. H., Xin L., et al., “Heterologous Desensitization of Opioid Receptors by Chemokines Inhibits Chemotaxis and Enhances the Perception of Pain,” Proceedings of the National Academy of Sciences of the United States of America 99, no. 16 (2002): 10276–10281, 10.1073/pnas.102327699. - DOI - PMC - PubMed
-
- Merighi S., Gessi S., Varani K., Fazzi D., Stefanelli A., and Borea P. A., “Morphine Mediates a Proinflammatory Phenotype via Mu‐Opioid Receptor‐PKCvarepsilon‐Akt‐ERK1/2 Signaling Pathway in Activated Microglial Cells,” Biochemical Pharmacology 86, no. 4 (2013): 487–496, 10.1016/j.bcp.2013.05.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

