Clinical evaluation and outcome in heart failure patients receiving chemotherapy with different anti-cancer agents
- PMID: 39801274
- PMCID: PMC12055377
- DOI: 10.1002/ehf2.15204
Clinical evaluation and outcome in heart failure patients receiving chemotherapy with different anti-cancer agents
Abstract
Background: The optimal strategy for modern chemotherapy should be based on a comprehensive approach for cancer patients with cardiovascular diseases. Therefore, cardio-oncology has received increasing attention owing to the cardiotoxic effects of anti-cancer therapies.
Objectives: We aimed to evaluate the clinical characteristics and outcomes of patients with heart failure (HF) who received chemotherapy compared with those of a matched cohort with HF who did not receive chemotherapy, using real-world HF data.
Methods: This study was based on the Diagnosis Procedure Combination (DPC) database of the Japanese Registry of All Cardiac and Vascular Diseases (JROAD). We identified 1 328 113 patients who were hospitalized for HF between April 2012 and March 2021. The propensity score (PS) was estimated using a logistic regression model, with chemotherapy as the dependent variable, and a clinically score-matched analysis of 11 532 patients with HF with or without chemotherapy. The primary endpoint was readmission.
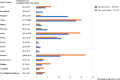
Results: Colon, lung, breast and prostate cancers accounted for >60% of all cancer types. After PS matching, readmission was significantly more frequently observed in patients with chemotherapy than those without [odds ratio (OR), 1.26; 95% confidence interval (CI) 1.17-1.36, P < 0.01]. In particular, treatment with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) (OR, 1.69; 95% CI 1.39-2.07), taxane (OR, 2.95; 95% CI 2.11-4.12), anthracyclines (OR, 1.86; 95% CI 1.19-2.90) and fluorouracil agents (OR, 1.65; 95% CI 1.18-2.30) caused a higher risk of readmission.
Conclusions: Medical providers need to monitor and follow-up patients with HF, depending on the characteristics of the anti-cancer agents and types of cancer.
Keywords: cardio‐oncology; chemotherapy; heart failure; readmission.
© 2024 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Tomiko Sunaga received personal fees from the Academic Subcommittee on Health Care and Pharmaceutical Sciences. Yoshitaka Iso received speaker fees from Novartis, Bayer, Toa Eiyo, Tsumura, Daiichi Sankyo, AstraZeneca and Viatris and received a joint research grant from NTT Communications. Mio Ebato received speaker fees from Novartis, Otsuka, Sanofi, Pfizer, Ono, Bayer, Sumitomo Pharma, AstraZeneca, Boehringer Ingelheim and Kowa and has received scholarship funds from Pfizer. Tsutomu Toshida received speaker fees from Daiichi Sankyo and Toa Eiyo. Shuichi Nawata received speaker fees from Pfizer, Daiichi Sankyo, Chugai, Bristol Myers Squibb and Qol and also received consulting fees from Afrac and payment for manuscript writing from Lilly. Hiroshi Suzuki received speaker fees from Novartis and Otsuka, scholarship funds from Abbott and Daiichi Sankyo, and a joint research grant from Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The Department of Hospital Pharmaceutics, School of Pharmacy, Showa University, received a contract for research from Ono Pharmaceutical Co., Ltd.
Figures

References
-
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768‐2801. doi:10.1093/eurheartj/ehw211 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

