Multicenter analysis of the incidence of in-stent stenosis following the deployment of flow-redirection endoluminal device in the treatment of intracranial aneurysms
- PMID: 39801312
- PMCID: PMC11726497
- DOI: 10.1177/15910199241304855
Multicenter analysis of the incidence of in-stent stenosis following the deployment of flow-redirection endoluminal device in the treatment of intracranial aneurysms
Abstract
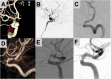
Introduction: The Flow Re-direction Endoluminal Device (FRED) is a novel flow diverter with a unique double stent design, with an inner stent composed of 48 nitinol wires, and an outer stent with 16 nitinol wires. It is designed for endovascular cerebral aneurysm treatment, although, limited data exist regarding in-stent stenosis (ISS) rates associated with FRED devices.
Methods: A registry encompassing two North American comprehensive stroke centers was the base of this study. We longitudinally assessed patients implanted with FRED devices, emphasizing baseline demographics, aneurysmal characteristics, procedural data, aneurysmal occlusion, and the incidence of ISS.
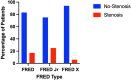
Results: In our cohort of 87 patients receiving 88 FRED devices, ISS occurred in 10.4% (9) of cases. Mild ISS (<50%) was noted in 8.0% (7) of patients, moderate ISS (50-75%) in 1.1% (1), and severe ISS (>75%) in 1.1% (1). Analysis indicated ISS in 17.0% (8) of patients with the FRED device and 5.7% (2) with the FRED-X device; all ISS cases in the FRED-X group were mild. Differences in ISS rates between device types were not significant (p = 0.122). Delayed thrombotic events were documented in 6.9% (6) of patients. Aneurysm occlusion rates, measured via the Raymond-Roy Scale (RRS), showed adequate occlusion (RRS 1 or 2) in 68.8% at 3 months, 74.6% at 6 months, and 89.3% at 12 months.
Conclusions: The study elucidates the efficacy and safety profile of FRED devices, presenting favorable aneurysm occlusion rates and low retreatment needs while underscoring the manageability of ISS.
Keywords: FRED; Flow redirection endoluminal device; cerebral aneurysm occlusion; endovascular treatment; in-stent stenosis; intracranial aneurysms.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
North American multicenter experience with the Flow Redirection Endoluminal Device in the treatment of intracranial aneurysms.J Neurosurg. 2022 Sep 9;138(4):933-943. doi: 10.3171/2022.7.JNS221371. Print 2023 Apr 1. J Neurosurg. 2022. PMID: 36087324
-
Lower-Ischemic-Risk Profile of Coated Flow Redirection Endoluminal Device X Compared With Uncoated Flow Redirection Endoluminal Device Flow Diverter in the Treatment of Unruptured Intracranial Aneurysms.Neurosurgery. 2024 Oct 8. doi: 10.1227/neu.0000000000003188. Online ahead of print. Neurosurgery. 2024. PMID: 39471102
-
Flow redirection endoluminal device (FRED) for treatment of intracranial aneurysms: A systematic review.Interv Neuroradiol. 2022 Jun;28(3):347-357. doi: 10.1177/15910199211027991. Epub 2021 Jun 30. Interv Neuroradiol. 2022. PMID: 34192977 Free PMC article.
-
Comparison of Flow-Redirection Endoluminal Device and Pipeline Embolization Device in the Treatment of Intracerebral Aneurysms.Neurosurgery. 2023 Jan 1;92(1):118-124. doi: 10.1227/neu.0000000000002148. Epub 2022 Sep 28. Neurosurgery. 2023. PMID: 36170173
-
Comparison of pipeline embolization device and flow redirection endoluminal device in the treatment of intracranial aneurysms: A systematic review and meta-analysis.Interv Neuroradiol. 2024 Jul 25:15910199241264345. doi: 10.1177/15910199241264345. Online ahead of print. Interv Neuroradiol. 2024. PMID: 39053432 Free PMC article. Review.
References
-
- Salem MM, Kvint S, Hendrix P, et al. The Pennsylvania postmarket multicenter experience with flow redirection endoluminal device. Neurosurgery 2022; 91: 280–285. - PubMed
LinkOut - more resources
Full Text Sources

