Stem cell mechanoadaptation. I. Effect of microtubule stabilization and volume changing stresses on cytoskeletal remodeling
- PMID: 39801500
- PMCID: PMC11719676
- DOI: 10.1063/5.0231273
Stem cell mechanoadaptation. I. Effect of microtubule stabilization and volume changing stresses on cytoskeletal remodeling
Abstract
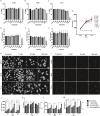
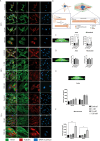
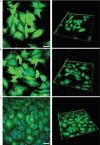
Here, we report on the first part of a two-part experimental series to elucidate spatiotemporal cytoskeletal remodeling, which underpins the evolution of stem cell shape and fate, and the emergence of tissue structure and function. In Part I of these studies, we first develop protocols to stabilize microtubules exogenously using paclitaxel (PAX) in a standardized model murine embryonic stem cell line (C3H/10T1/2) to maximize comparability with previously published studies. We then probe native and microtubule-stabilized stem cells' capacity to adapt to volume changing stresses effected by seeding at increasing cell densities, which emulates local compression and tissue template formation during development. Within the concentration range of 1-100 nM, microtubule-stabilized stem cells maintain viability and reduce proliferation. PAX stabilization of microtubules is associated with increased cell volume as well as flattening of the cell and nucleus. Compared to control cells, microtubule-stabilized cells exhibit thick, bundled microtubules and highly aligned, thicker and longer F-actin fibers, corresponding to an increase in the Young's modulus of the cell. Both F-actin and microtubule concentration increase with increasing PAX concentration, whereby the increase in F-actin is more prominent in the basal region of the cell. The corresponding increase in microtubule is observed more globally across the apical and basal region of the cell. Seeding at increasing target densities induces local compression on cells. This increase in local compression modulates cell volume and concomitant increases in F-actin and microtubule concentration to a greater degree than microtubule stabilization via PAX. Cells seeded at high density exhibit higher bulk modulus than corresponding cells seeded at low density. These data demonstrate the capacity of stem cells to adapt to an interplay of mechanical and chemical cues, i.e., respective compression and exogenous microtubule stabilization; the resulting cytoskeletal remodeling manifests as evolution of mechanical properties relevant to development of multicellular tissue constructs.
© 2025 Author(s).
Conflict of interest statement
M.L.K.T. has co-founded startup companies to commercialize the intellectual property she and her collaborators developed and patent protected over the past decades. The topic of the current manuscript is fundamental in nature and not directly related to M.L.K.T.'s innovation translation and commercialization projects.
Figures




Similar articles
-
Stem cell mechanoadaptation. II. Microtubule stabilization and substrate compliance effects on cytoskeletal remodeling.APL Bioeng. 2025 Jan 7;9(1):016103. doi: 10.1063/5.0231287. eCollection 2025 Mar. APL Bioeng. 2025. PMID: 39801501 Free PMC article.
-
Structure-function relationships in the stem cell's mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure.Mol Cell Biomech. 2011 Dec;8(4):297-318. Mol Cell Biomech. 2011. PMID: 22338708 Free PMC article.
-
Structure-function relationships in the stem cell's mechanical world A: seeding protocols as a means to control shape and fate of live stem cells.Mol Cell Biomech. 2011 Dec;8(4):275-96. Mol Cell Biomech. 2011. PMID: 22338707
-
Role of microtubule-associated proteins in the control of microtubule assembly.Physiol Rev. 1995 Oct;75(4):835-64. doi: 10.1152/physrev.1995.75.4.835. Physiol Rev. 1995. PMID: 7480164 Review.
-
Cytoskeletal social networking in the growth cone: How +TIPs mediate microtubule-actin cross-linking to drive axon outgrowth and guidance.Cytoskeleton (Hoboken). 2016 Sep;73(9):461-76. doi: 10.1002/cm.21272. Epub 2016 Feb 8. Cytoskeleton (Hoboken). 2016. PMID: 26783725 Free PMC article. Review.
References
-
- Zimmermann J. A. and Knothe Tate M. L., “ Structure–function relationships in the stem cell's mechanical world A: Seeding protocols as a means to control shape and fate of live stem cells,” Mol. Cell. Biomech. 8(4), 275–296 (2011). - PubMed
LinkOut - more resources
Full Text Sources

