This is a preprint.
Abnormal redox balance at membrane contact sites causes axonopathy in GDAP1-related Charcot-Marie-Tooth disease
- PMID: 39801517
- PMCID: PMC11722552
- DOI: 10.21203/rs.3.rs-5682984/v1
Abnormal redox balance at membrane contact sites causes axonopathy in GDAP1-related Charcot-Marie-Tooth disease
Abstract
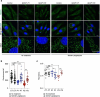
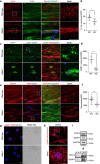
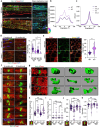
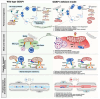
Pathogenic variants of GDAP1 cause Charcot-Marie-Tooth disease (CMT), an inherited neuropathy characterized by axonal degeneration. GDAP1, an atypical glutathione S-transferase, localizes to the outer mitochondrial membrane (OMM), regulating this organelle's dynamics, transport, and membrane contact sites (MCSs). It has been proposed that GDAP1 functions as a cellular redox sensor. However, its precise contribution to redox homeostasis remains poorly understood, as does the possible redox regulation at mitochondrial MCSs. Given the relationship between the peroxisomal redox state and overall cellular redox balance, we investigated the role of GDAP1 in peroxisomal function and mitochondrial MCSs maintenance by using high-resolution microscopy, live cell imaging with pH-sensitive fluorescent probes, and transcriptomic and lipidomic analyses in the Gdap1 -/- mice and patient-derived fibroblasts. We demonstrate that GDAP1 deficiency disrupts mitochondria-peroxisome MCSs and leads to peroxisomal abnormalities, which are reversible upon pharmacological activation of PPARγ or glutathione supplementation. These results identify GDAP1 as a new tether of mitochondria-peroxisome MCSs that maintain peroxisomal number and integrity. The supply of glutathione (GSH-MEE) or GDAP1 overexpression suffices to rescue these MCSs. Furthermore, GDAP1 may regulate the redox state within the microdomain of mitochondrial MCSs, as suggested by decreased pH at mitochondria-lysosome contacts in patient-derived fibroblasts, highlighting the relationship between GDAP1 and redox-sensitive targets. Finally, in vivo analysis of sciatic nerve tissue in Gdap1 -/- mice revealed significant axonal structural abnormalities, including nodes of Ranvier disruption and defects in the distribution and morphology of mitochondria, lysosomes, and peroxisomes, emphasizing the importance of GDAP1 in sustaining axon integrity in the peripheral nervous system. Taken together, this study positions GDAP1 as a multifunctional protein that mediates mitochondrial interaction with cellular organelles of diverse functions, contributes to redox state sensing, and helps maintain axonal homeostasis. In addition, we identify PPAR as a novel therapeutic target, based on knowledge of the underlying pathogenetic mechanisms.
Conflict of interest statement
Competing Interests Statement The authors declare no competing interests.
Figures

Similar articles
-
Mitochondria-lysosome membrane contacts are defective in GDAP1-related Charcot-Marie-Tooth disease.Hum Mol Genet. 2021 Jan 21;29(22):3589-3605. doi: 10.1093/hmg/ddaa243. Hum Mol Genet. 2021. PMID: 33372681 Free PMC article.
-
Differential effects of Mendelian GDAP1 clinical variants on mitochondria-lysosome membrane contacts sites.Biol Open. 2023 Apr 15;12(4):bio059707. doi: 10.1242/bio.059707. Epub 2023 Apr 3. Biol Open. 2023. PMID: 36912213 Free PMC article.
-
Mitochondrial Dynamics and Mitochondria-Lysosome Contacts in Neurogenetic Diseases.Front Neurosci. 2022 Jan 31;16:784880. doi: 10.3389/fnins.2022.784880. eCollection 2022. Front Neurosci. 2022. PMID: 35177962 Free PMC article.
-
Calcium Deregulation and Mitochondrial Bioenergetics in GDAP1-Related CMT Disease.Int J Mol Sci. 2019 Jan 18;20(2):403. doi: 10.3390/ijms20020403. Int J Mol Sci. 2019. PMID: 30669311 Free PMC article. Review.
-
Mitochondrial dynamics and inherited peripheral nerve diseases.Neurosci Lett. 2015 Jun 2;596:66-77. doi: 10.1016/j.neulet.2015.04.001. Epub 2015 Apr 3. Neurosci Lett. 2015. PMID: 25847151 Review.
Cited by
-
Peroxisome dynamics and inter-organelle interactions in neuronal health and disease.Front Mol Neurosci. 2025 Jun 20;18:1603632. doi: 10.3389/fnmol.2025.1603632. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40621503 Free PMC article. Review.
References
-
- Nelis E., et al. Mutations in GDAP1: autosomal recessive CMT with demyelination and axonopathy. Neurology 59, 1865–1872 (2002). - PubMed
-
- Cuesta A., et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet 30, 22–25 (2002). - PubMed
-
- Sevilla T., et al. Clinical, electrophysiological and morphological findings of Charcot-Marie-Tooth neuropathy with vocal cord palsy and mutations in the GDAP1 gene. Brain 126, 2023–2033 (2003). - PubMed
-
- Baxter R.V., et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet 30, 21–22 (2002). - PubMed
-
- Pedrola L., et al. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum Mol Genet 14, 1087–1094 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

