Real-World Treatment Outcomes of an Artificial Tear Containing Arabinogalactan, Hyaluronic Acid and Trehalose Among Subjects with Dry Eye
- PMID: 39801568
- PMCID: PMC11724676
- DOI: 10.2147/OPTH.S480668
Real-World Treatment Outcomes of an Artificial Tear Containing Arabinogalactan, Hyaluronic Acid and Trehalose Among Subjects with Dry Eye
Abstract
Purpose: To assess the efficacy, adherence, and tolerability of a new artificial tear based on arabinogalactan, hyaluronic acid, and trehalose in a population with dry eye disease (DED).
Methods: A retrospective, real-world, post-marketing study identified 96 adult patients (aged 18-80 years) with signs and symptoms of dry eye. These patients received fixed combination therapy with eye drops containing arabinogalactan, hyaluronic acid, and trehalose at various dosing schedules. The data for this study were collected from April 2022 to June 2023. Patients underwent evaluation at baseline (T0) and after a follow-up period of two-three months (T1) using a patient-reported questionnaire.
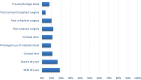
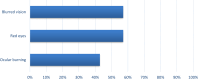
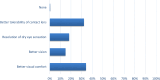
Results: In 96 adult patients (71 women and 25 men) with dry eye due to various conditions, the results indicated a 98% positive response to therapy. This response included improvements in vision (13%), comfort (39%), redness (13%), itching (16%), photophobia (4%), and tearing (14%). Additionally, 61% of the patients experienced 1-2 hours of comfort following instillation.
Conclusion: This real-life post-marketing study demonstrated clinical improvement of signs and symptoms in patients with dry eye disease using a new artificial tear medical device based on arabinogalactan, hyaluronic acid, and trehalose.
Keywords: arabinogalactan; dry eye; hyaluronic acid; post-marketing study; real-world study; trehalose.
© 2025 Bedei et al.
Conflict of interest statement
The authors declare no conflicts of interest that could have influenced the work reported in this paper.
Figures
Similar articles
-
Improving ocular surface comfort in contact lens wearers.Cont Lens Anterior Eye. 2022 Jun;45(3):101544. doi: 10.1016/j.clae.2021.101544. Epub 2021 Nov 25. Cont Lens Anterior Eye. 2022. PMID: 34840071
-
Efficacy of artificial tears containing trehalose and hyaluronic acid for dry eye disease in women aged 42-54 versus ≥ 55 years.Cont Lens Anterior Eye. 2023 Aug;46(4):101845. doi: 10.1016/j.clae.2023.101845. Epub 2023 Apr 26. Cont Lens Anterior Eye. 2023. PMID: 37117131
-
Tear Film Stabilization and Symptom Improvement in Dry Eye Disease: The Role of Hyaluronic Acid and Trehalose Eyedrops versus Carmellose Sodium.J Clin Med. 2023 Oct 20;12(20):6647. doi: 10.3390/jcm12206647. J Clin Med. 2023. PMID: 37892784 Free PMC article.
-
Safe and Effective Management of Dry Eye Symptoms with Hydroxypropyl Guar and Hyaluronic Acid Dual-Polymer Lubricating Eye Drops: A Review of Preclinical and Clinical Studies.Clin Ophthalmol. 2023 Dec 13;17:3883-3898. doi: 10.2147/OPTH.S428725. eCollection 2023. Clin Ophthalmol. 2023. PMID: 38105908 Free PMC article. Review.
-
Assessing the therapeutic role of trehalose and hyaluronic acid: implications for patient care.Int Ophthalmol. 2024 Oct 1;44(1):398. doi: 10.1007/s10792-024-03308-1. Int Ophthalmol. 2024. PMID: 39352586
References
LinkOut - more resources
Full Text Sources
Miscellaneous