Early Change in C-Reactive Protein and Venous Thromboembolism in Patients Treated With Immune Checkpoint Inhibitors
- PMID: 39801631
- PMCID: PMC11711807
- DOI: 10.1016/j.jaccao.2024.09.007
Early Change in C-Reactive Protein and Venous Thromboembolism in Patients Treated With Immune Checkpoint Inhibitors
Abstract
Background: Patients with cancer treated with immune-checkpoint inhibitors (ICIs) have a substantial risk of venous thromboembolism (VTE). The association between ICI-induced inflammation and hypercoagulability is unclear, and no biomarkers currently exist to stratify VTE risk.
Objectives: The authors sought to determine the association between the early changes in C-reactive protein (CRP) after ICI initiation and the risk of VTE.
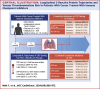
Methods: This retrospective cohort study included patients with cancer initiating ICI therapy from 2 academic cancer centers, serving as discovery and external validation cohorts. Patients were stratified based on CRP trajectories during the first 3 months of ICI treatment, with a CRP rise defined as a 2-fold increase from baseline. Patients were followed for VTE for the duration of ICI therapy, and competing risk and time-dependent analyses were used.
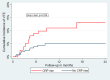
Results: A total of 822 patients were included. In the discovery cohort (n = 405), the cumulative VTE incidence in patients with a CRP rise (n = 159, 39.3%) was 19.9% (95% CI: 8.4%-34.8%), compared with 8.6% (3.1%-17.6%) in those without a CRP rise. After adjusting for key patient- and cancer-specific confounders, the subdistribution HR for VTE in patients with a CRP rise was 2.64 (95% CI: 1.06-6.62). This was confirmed in the external validation cohort (n = 417; subdistribution HR: 2.25; 95% CI: 1.03-4.94), with VTE incidences of 22.9% (95% CI: 9.7%-39.3%) in patients with a CRP rise and 10.8% (95% CI: 7.4%-15.1%) in those without. The association between CRP rise and VTE risk was confirmed in a time-dependent analysis and was consistent after adjusting for disease progression as a potential time-dependent confounder.
Conclusions: Early CRP changes during ICI therapy are associated with an increased risk of VTE, suggesting a potential association between ICI-induced inflammation and hypercoagulability. CRP trajectories may serve as a biomarker for ICI-associated VTE.
Keywords: C-reactive protein; cancer; immune checkpoint inhibitors; thrombosis; venous thromboembolism.
© 2024 The Authors.
Conflict of interest statement
This project was supported by the Society of Thrombosis and Haemostasis Research (Gesellschaft für Thrombose- und Hämostaseforschung, GTH) Early Career Research Grant 2021. The AUTRICHE-registry was supported by a research grant from AstraZeneca GmbH, Bristol-Myers Squibb GesmbH (BMS) and Roche Austria. The funding body had no role in the design, analysis, and publication of this study. Dr Moik has received travel/congress support from Novartis; and honoraria for lectures from Servier and Bristol Myers Squibb. Dr Riedl has received honoraria for lectures from BMS and MSD. Dr Barth has received travel/congress support from EISAI, Lilly, Bristol Myers Squibb, and MSD; honoraria for consulting or advisory boards from Roche, EISAI, and MSD; and honoraria for lectures from Ipsen unrelated to the submitted work. Dr Hoeller has received speaker honoraria from Amgen, BMS, MSD, Novartis, and Roche; and participated on advisory boards for Amgen, Astra Zeneca, BMS, Inzyte, MSD, Novartis, Pierre Fabre, and Roche. Dr Fuereder has received honoraria from MSD, Merck Darmstadt, Roche, BMS, Accord, Sanofi, and Boehringer Ingelheim; and participated on advisory boards for MSD, Merck Darmstadt, Amgen, Pfizer, and Sanofi. Dr L. Ay has received personal fees for lectures and participation on advisory boards from Amgen, BMS, MSD, Roche, Sandoz, and Astra Zeneca. Dr Pabinger has received honoraria for lectures and advisory board meetings from Bayer AG, Boehringer Ingelheim, Daiichi Sankyo, and BMS/ Pfizer. Dr Richtig has received honoraria from Amgen, Bayer, Bristol Myers Squibb, Delcath, Merck Sharp Dohme, Merck, Novartis, Pierre Fabre, Roche, and Sanofi; has had a consulting or advisory role for Amgen, Bayer, Bristol Myers Squibb, Merck Sharp & Dohme, Merck, Novartis, and Pierre Fabre; has served on the speakers bureau for Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Merck, Novartis, Pierre Fabre, and Sanofi; and has received institutional research funding from Amgen, Bristol Myers Squibb, Delcath, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche, and Curevac. Dr Jost has had a consulting or advisory role, and received honoraria, research funding, and/or travel/accommodation expenses, from AstraZeneca, Bayer, Boehringer, Novartis, Pfizer, Servier, Roche, BMS and Celgene, Pierre Fabre, Janssen/Johnson & Johnson, MSD, Merck, Sanofi/Aventis, Ipsen, and Amgen. Dr Gerger has received honoraria for lectures and advisory board participation from Merck Sharp & Dohme, Bristol Myers Squibb, Roche, and AstraZeneca. Dr Terbuch has received honoraria for lectures and advisory board participation from Merck Sharp & Dohme, Bristol Myers Squibb, Roche, and AstraZeneca. Dr Preusser has received honoraria for lectures, consultation, or advisory board participation from Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, AstraZeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dohme, Tocagen, Adastra, Gan & Lee Pharmaceuticals, Janssen, Servier, Miltenyi, Bohringer Ingelheim, Telix, and Medscape. Dr C. Ay has received personal fees for lectures and/or participation in advisory boards from Bayer, Daiichi Sankyo, BMS/Pfizer Alliance, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

