SGLT2i and Primary Prevention of Cancer Therapy-Related Cardiac Dysfunction in Patients With Diabetes
- PMID: 39801650
- PMCID: PMC11711834
- DOI: 10.1016/j.jaccao.2024.08.001
SGLT2i and Primary Prevention of Cancer Therapy-Related Cardiac Dysfunction in Patients With Diabetes
Abstract
Background: Specific cancer treatments can lead to cancer therapy-related cardiac dysfunction (CTRCD). Sodium glucose cotransporter-2 inhibitors (SGLT2is) can potentially prevent these cardiotoxic effects.
Objectives: This study sought to determine whether SGLT2i use is associated with a lower incidence of CTRCD in patients with type 2 diabetes mellitus (T2DM) and cancer, exposed to potentially cardiotoxic antineoplastic agents, and without a prior documented history of cardiomyopathy or heart failure.
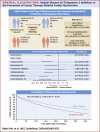
Methods: We conducted a retrospective analysis of patients aged ≥18 years within the TriNetX database with T2DM, cancer, exposure to cardiotoxic therapies, and no prior documented history of cardiomyopathy or heart failure. Patients were categorized by SGLT2i use. After propensity score matching, outcomes were compared over 12 months using Cox proportional HRs. Subgroup analyses focusing on different cancer therapy classes were performed.
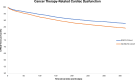
Results: The study included 8,675 propensity-matched patients in each cohort (mean age = ∼65 years, 42% females, 71% White, ∼19% gastrointestinal malignancy, and ∼25% anthracyclines). Patients prescribed SGLT2is had a lower risk of developing CTRCD (HR: 0.76: 95% CI: 0.69-0.84). SGLT2is also reduced heart failure exacerbations (HR: 0.81; 95% CI: 0.72-0.90), all-cause mortality (HR: 0.67; 95% CI: 0.61-0.74), and all-cause hospitalizations/emergency department visits (HR: 0.93; 95% CI: 0.89-0.97). Subgroup analyses also demonstrated reduced CTRCD risk across various classes of cancer therapies in patients prescribed SGLT2is.
Conclusions: SGLT2i administration was associated with a significantly decreased risk of developing CTRCD in patients with T2DM and cancer.
Keywords: CTRCD; antineoplastic therapy; cardiomyopathy; primary prevention; sodium glucose co-transporter 2 inhibitors.
© 2024 The Authors.
Conflict of interest statement
Dr Yang has received research funding from CSL Behring (nonrelevant), Boehringer Ingelheim and Eli and Lilly, Amgen (nonrelevant), and Bristol Meyers Squibb (nonrelevant); and has received consulting fees from Xencor (nonrelevant). Dr Deswal has received consulting fees from Bayer. Dr Herrmann has received consulting fees from Pfizer, Astellas, and AstraZeneca; and has received grant funding from the National Institutes of Health (RO1 CA233610) and the Miami Heart Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures