Bifidobacterium longum RAPO Attenuates Dermal and Pulmonary Fibrosis in a Mouse Model of Systemic Sclerosis through Macrophage Modulation and Growth of Short-Chain Fatty Acid Producers
- PMID: 39801739
- PMCID: PMC11711128
- DOI: 10.4110/in.2024.24.e41
Bifidobacterium longum RAPO Attenuates Dermal and Pulmonary Fibrosis in a Mouse Model of Systemic Sclerosis through Macrophage Modulation and Growth of Short-Chain Fatty Acid Producers
Abstract
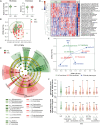
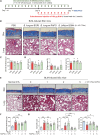
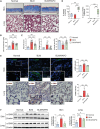
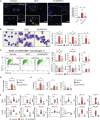
Systemic sclerosis (SSc) is a complex autoimmune disease with an unclear etiology and no effective treatments. Recent research has suggested involvement of the microbiome in SSc pathogenesis. This study aimed to identify specific microbial species associated with SSc and explore their therapeutic potential. Serum Abs against 384 intestinal microbial species revealed a significant depletion in Abs against Bifidobacterium longum in patients with SSc compared to healthy controls. In a bleomycin-induced SSc mouse model, oral administration of B. longum strain RAPO attenuated skin and lung fibrosis, accompanied by reduced infiltration of inflammatory monocytes/macrophages and downregulation of pro-inflammatory cytokines and chemoattractant Ccl2 genes in lymph nodes and fibrotic tissues. B. longum RAPO treatment restored fecal microbial diversity and augmented short-chain fatty acid (SCFA)-producing bacteria in the gut, leading to increased fecal butyrate levels and upregulated SCFA receptor Gpr41 in the mesenteric lymph node. In vitro, B. longum RAPO and its culture supernatant suppressed the expressions of pro-inflammatory cytokine genes in macrophages and inhibited myofibroblast differentiation in fibroblasts. These findings highlight the probiotic potential of B. longum RAPO in preventing tissue fibrosis by modulating macrophage activity and promoting the growth of SCFA-producing bacteria, underscoring the therapeutic potential of microbial modulation in SSc.
Keywords: Bacterial antibodies; Bifidobacterium longum; Fibrosis; Short-chain fatty acid; Systemic sclerosis.
Copyright © 2024. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures


References
-
- Tsou PS, Varga J, O’Reilly S. Advances in epigenetics in systemic sclerosis: molecular mechanisms and therapeutic potential. Nat Rev Rheumatol. 2021;17:596–607. - PubMed
LinkOut - more resources
Full Text Sources

