The mechano-electric feedback mediates the dual effect of stretch in mouse sinoatrial tissue
- PMID: 39802174
- PMCID: PMC11708250
- DOI: 10.1016/j.jmccpl.2023.100042
The mechano-electric feedback mediates the dual effect of stretch in mouse sinoatrial tissue
Abstract
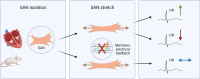
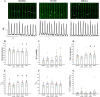
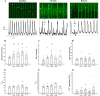
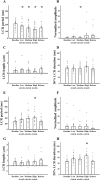
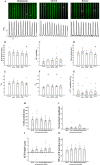
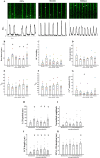
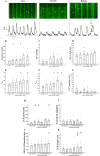
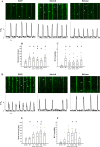
The sinoatrial node (SAN) is the primary heart pacemaker. The automaticity of SAN pacemaker cells is regulated by an integrated coupled-clock system. The beat interval (BI) of SAN, and its primary initiation location (inferior vs. superior) are determined by mutual entrainment among pacemaker cells and interaction with extrinsic effectors, including increased venous return which stretches the SAN. We aim to understand the mechanisms that link stretch to changes in BI and to heterogeneity of BI in the SAN. Isolated SAN tissues of C57BL/6 mice were gradually stretched to different degrees [(low (5-10 % lengthening), medium (10-20 %), and high (20-40 %))] using motor controlled with a custom-made Arduino software. Recordings were acquired 30 s following each level of step. In 8/15 tissues, stretch led to a positive chronotropic response, while in 7/15 tissues, a negative chronotropic response was observed. In the positive chronotropic response group, BI was shortened in parallel to shortening of the local Ca2+ release (LCR) period, a readout of the degree of clock coupling. In the negative chronotropic response group, in parallel to a prolongation of BI and LCR period, an unsynchronized firing rate was observed among the cells upon application of stretch. Eliminating the mechano-electrical feedback by addition of blebbistatin disabled the stretch-induced chronotropic effect. Reduction of the sarcoplasmic reticulum Ca2+ levels, which mediates the mechano-electrical feedback, by addition of cyclopiazonic acid disabled the dual effect of stretch on SAN function and BI heterogeneity. Thus, the mechano-electric feedback mediates the dual effect of stretch in mouse SAN tissue.
Keywords: Calcium cycling; Computational model; Ion channels; Sinoatrial node; Stretch.
© 2023 The Authors.
Conflict of interest statement
None.
Figures
References
-
- Boyett M.R., Honjo H., Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. [doi:S0008-6363(00)00135-8 [pii]] - PubMed
-
- Bychkov R., Juhaszova M., Tsutsui K., Coletta C., Stern M.D., Maltsev V.A., et al. Synchronized cardiac impulses emerge from heterogeneous local calcium signals within and among cells of pacemaker tissue. JACC Clin Electrophysiol. 2020;6:907–931. doi: 10.1016/J.JACEP.2020.06.022. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

