Impact of Granulocyte Colony-Stimulating Factor (G-CSF) on Clinical Outcomes in Allogeneic Hematopoietic Cell Transplantation: Does Speeding Up Neutrophil Engraftment Make a Difference?
- PMID: 39802196
- PMCID: PMC11723673
- DOI: 10.1097/TXD.0000000000001753
Impact of Granulocyte Colony-Stimulating Factor (G-CSF) on Clinical Outcomes in Allogeneic Hematopoietic Cell Transplantation: Does Speeding Up Neutrophil Engraftment Make a Difference?
Abstract
Background: Despite decades of post-allogeneic hematopoietic cell transplantation (HCT) growth factor utilization, its role remains undefined, leading to ongoing debates and research. The theoretical impacts of growth factors have been challenged in numerous studies.
Methods: In this retrospective cohort study conducted at the Princess Margaret Cancer Centre, we analyzed the clinical outcomes of 509 patients who underwent allogeneic HCT between May 1, 2019, and May 31, 2022. This study aimed to assess the impact of granulocyte colony-stimulating factor (G-CSF) administration posttransplantation on neutrophil and platelet engraftment, incidence of bloodstream infections (BSIs), graft-versus-host disease, engraftment syndrome (ES), and survival metrics including overall survival, nonrelapse mortality, and graft-versus-host disease-free/relapse-free survival.
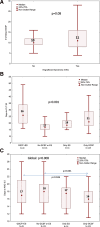
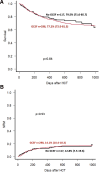
Results: Our findings indicate that G-CSF administration expedited neutrophil engraftment (16 versus 18 d, P = 0.009) and was associated with a decreased incidence of BSI (9.4% versus 31.3%, P = 0.014). However, this benefit was counterbalanced by a significant delay in platelet engraftment (21 versus 17 d, P < 0.001). Multivariate logistic regression analysis identified mismatched donors (odds ratio, 1.72; 95% confidence interval, 1.03-2.88; P = 0.038) and the duration of G-CSF therapy (odds ratio, 1.04; 95% confidence interval, 1.00-1.09; P = 0.038) as independent predictors for the development of ES. Despite these hematological impacts, there was no observed advantage in overall survival, nonrelapse mortality, or graft-versus-host disease-free/relapse-free survival among patients who received G-CSF compared with those who did not.
Conclusions: Although G-CSF post-HCT expedited neutrophil engraftment and reduced BSI risk, it did not result in a survival advantage. The association with ES necessitates careful consideration.
Copyright © 2025 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Figures


References
-
- Ringdén OTH, Le Blanc K, Remberger M. Granulocyte and granulocyte-macrophage colony-stimulating factors in allografts: uses, misuses, misconceptions, and future applications. Exp Hematol. 2005;33:505–512. - PubMed
-
- Hägglund H Ringdén O Man SO¨, et al. A prospective randomized trial of Filgrastim (r-MetHuG-CSF) given at different times after unrelated bone marrow transplantation. 1999;24:831–836. - PubMed
-
- Stinson TJ, Adams JR, Bishop MR, et al. Economic analysis of a phase III study of G-CSF vs placebo following allogeneic blood stem cell transplantation. Bone Marrow Transplant. 2000;26:663–666. - PubMed
-
- Bishop MR, Tarantolo SR, Geller RB, et al. A randomized, double-blind trial of Filgrastim (granulocyte colony-stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood. 96:80–85. - PubMed
-
- Przepiorka D, Smith TL, Folloder J, et al. Controlled trial of Filgrastim for acceleration of neutrophil recovery after allogeneic blood stem cell transplantation from human leukocyte antigen-matched related donors. Blood. 2001;97(11):3405-3410. - PubMed
LinkOut - more resources
Full Text Sources

