Clinical Outcomes and Donor-specific Antibody Rebound 5 y After Kidney Transplant Enabled by Imlifidase Desensitization
- PMID: 39802198
- PMCID: PMC11723687
- DOI: 10.1097/TXD.0000000000001752
Clinical Outcomes and Donor-specific Antibody Rebound 5 y After Kidney Transplant Enabled by Imlifidase Desensitization
Abstract
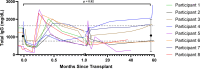
Background: Imlifidase is an IgG-cleaving endopeptidase conditionally approved in Europe for desensitization of highly sensitized patients before kidney transplantation. We present 5-y outcomes and donor-specific antibody (DSA) levels for clinical trial participants from a single site who received imlifidase for desensitization before incompatible transplantation (NCT02790437).
Methods: Imlifidase was administered up to 24 h before living or deceased donor kidney transplantation. DSAs were monitored before transplantation, at days 7 and 28, and at 5 y posttransplant.
Results: At 5 y, 7 of 8 participants were alive. One of these 7 had suboptimal graft function secondary to donor-derived disease but remained dialysis independent. Three participants had antibody-mediated rejection (AMR), which occurred in the first 30 d in all cases and was successfully treated. No new episodes of suspected or biopsy-proven AMR occurred after 30 d posttransplant. Seven participants had DSA rebound. DSAs commonly persisted 5 y posttransplant, although they were generally lower strength compared with pre-imlifidase. Dilution studies of sensitized serum enabled the identification of lower AMR risk phenotypes for persisting DSAs. Severe and/or opportunistic infections were not observed at greater than expected frequency.
Conclusions: Five-year outcomes of imlifidase-enabled incompatible transplants are overall favorable. DSA rebound is common, but antibody strength lessens in the long term, and longitudinally persisting DSAs did not lead to premature graft failure.
Copyright © 2025 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Figures





References
-
- Fidler SJ, Irish AB, Lim W, et al. . Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol. 2013;28:148–153. - PubMed
-
- Hönger G, Hopfer H, Arnold ML, et al. . Pretransplant IgG subclasses of donor-specific human leukocyte antigen antibodies and development of antibody-mediated rejection. Transplantation. 2011;92:41–47. - PubMed
-
- Riethmüller S, Ferrari-Lacraz S, Müller MK, et al. . Donor-specific antibody levels and three generations of crossmatches to predict antibody-mediated rejection in kidney transplantation. Transplantation. 2010;90:160–167. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

