Impact of an Oncology Clinical Pharmacist Intervention on Clinical Trial Enrollment in The US Oncology Network's MYLUNG Consortium
- PMID: 39802530
- PMCID: PMC11715463
- DOI: 10.6004/jadpro.2024.15.8.7
Impact of an Oncology Clinical Pharmacist Intervention on Clinical Trial Enrollment in The US Oncology Network's MYLUNG Consortium
Abstract
Introduction: The Molecularly Informed Lung Cancer Treatment in a Community Cancer Network: A Pragmatic Consortium™ (MYLUNG) clinical trial platform aims to advance the use of precision medicine in patients with non-small cell lung cancer through a series of prospective and iterative clinical trials. Timely patient accrual onto oncology clinical trials is a known practice challenge and impaired accrual rates can lead to premature trial closure or properly powered trial outcomes. The US Oncology Network recently implemented a clinical pharmacist (ClinReview) initiative to provide remote clinical services to screen patients for enrollment onto MYLUNG Protocol 2. This study aims to evaluate the effect of the remote clinical pharmacist intervention on study enrollment rates.
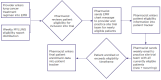
Methods: An oncology-trained clinical pharmacist remotely reviewed systemic chemotherapy treatment orders during normal workflow and, in addition, a weekly custom recruitment report within six community Network practices (149 physicians). The pharmacist identified, screened, and assisted with the communication regarding eligible patients for enrollment. The onsite research team received timely and relevant patient data to facilitate expedited enrollment. Enrollment and intervention data were tracked to monitor the impact of the pharmacist intervention. Monthly enrollment was evaluated using a paired t-test.
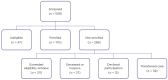
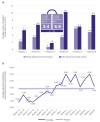
Results: Over 8 months, the pharmacist screened 506 potentially eligible patients; 34% were enrolled. Average monthly enrollment was significantly greater following the ClinReview intervention (3.4 vs. 6.6 patients/month; p = .02). Among the 289 patients not enrolled, 73% exceeded their eligibility window, 9% died or enrolled into hospice, 4% declined participation, and 13% transferred care or were treated at outside facilities.
Conclusions: Incorporating an oncology clinical pharmacist into the clinical research team was associated with improved clinical trial enrollment. Validation of the effect of multidisciplinary interventions across a broader spectrum of differentially resourced oncology practices will be conducted within future MYLUNG iterations.
© 2024 BroadcastMed LLC.
Conflict of interest statement
Amgen, AstraZeneca, Eli Lilly, Genentech, Janssen, and Mirati are research partners of MYLUNG Consortium. Dr. Coleman has received grants or contracts from AstraZeneca, Clovis, Genelux, Genmab, Merck, Immunogen, and Roche/Genentech; consulting fees from Agenus, Alkermes, AstraZeneca, Clovis, Deciphera, Genelux, Genmab, GSK, Immunogen, OncoQuest, Onxerna, Regeneron, Roche/Genentech, Novocure, Merck, and AbbVie; payment or honoraria from AstraZeneca, Clovis, Roche/Genentech, and Merck; and participated on a data safety monitoring board or advisory board for VBL Therapeutics. Dr. Butrynski has received consulting fees from Boehringer Ingelheim. Dr. Jotte has received consulting fees from Bristol Myers Squibb and Roche/Genentech. Dr. Evangelist has received consulting fees from AstraZeneca and Takeda. Dr. Waterhouse has received consulting fees from Bristol Myers Squibb, AZTherapies, AbbVie, Amgen, McGivney Global Advisors, Janssen Oncology, Seattle Genetics, Jazz Pharmaceuticals, Exelixis, Eisai, EMD Serono, Merck, Pfizer, Mirati Therapeutics, and Regeneron/Sanofi; received payment or honoraria from Bristol Myers Squibb, Janssen Oncology, Merck, and AstraZeneca; and support for travel from Bristol Myers Squibb. The remaining authors have no conflicts of interest to disclose.
Figures
References
-
- American Cancer Society Cancer Action Network. (2019). Barriers to patient enrollment in therapeutic clinical trials for cancer. https://www.fightcancer.org/policy-resources/barriers-patient-enrollment...
LinkOut - more resources
Full Text Sources
