This is a preprint.
PET/CT guided tuberculosis treatment shortening: a randomized trial
- PMID: 39802795
- PMCID: PMC11722446
- DOI: 10.1101/2024.10.03.24314723
PET/CT guided tuberculosis treatment shortening: a randomized trial
Abstract
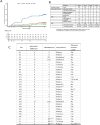
Six months of chemotherapy using current agents is standard of care for pulmonary, drug-sensitive tuberculosis (TB), even though some are believed to be cured more rapidly and others require longer therapy. Understanding what factors determine the length of treatment required for durable cure in individual patients would allow individualization of treatment durations, provide better clinical tools to determine the of appropriate duration of new regimens, as well as reduce the cost of large Phase III studies to determine the optimal combinations to use in TB control programs. We conducted a randomized clinical trial in South Africa and China that recruited 704 participants with newly diagnosed, drug-sensitive pulmonary tuberculosis and stratified them based on radiographic disease characteristics as assessed by FDG PET/CT scan readers. Participants with less extensive disease (N=273) were randomly assigned to complete therapy after four months or continue receiving treatment for six months. Amongst participants who received four months of therapy, 17 of 141 (12.1%) experienced unfavorable outcomes compared to only 2 of 132 (1.5%) who completed six months of treatment (treatment success 98.4% in B, 86.7% in C (difference -11.7%, 95% CI, -18.2%, -5.3%)). In the non-randomized arm that included participants with more extensive disease, only 8 of 248 (3.2%) experienced unfavorable outcomes. Total cavity volume and total lesion glycolysis at week 16 were significantly associated with risk of unfavorable outcome in the randomized participants. Based on PET/CT scans at TB recurrence, bacteriological relapses (confirmed by whole genome sequencing) predominantly occurred in the same active cavities originally present at baseline. Automated segmentation of the serial PET/CT scans was later performed, and machine-learning was used to classify participants according to their likelihood of relapse, allowing the development of predictive models with good performance based on CT, PET, microbiological and clinical characteristics. These results open the possibility for more efficient studies of future TB treatment regimens.
Figures


References
-
- Nahid P., Dorman S. E., Alipanah N., Barry P. M., Brozek J. L., Cattamanchi A., Chaisson L. H., Chaisson R. E., Daley C. L., Grzemska M., Higashi J. M., Ho C. S., Hopewell P. C., Keshavjee S. A., Lienhardt C., Menzies R., Merrifield C., Narita M., O'Brien R., Peloquin C. A., Raftery A., Saukkonen J., Schaaf H. S., Sotgiu G., Starke J. R., Migliori G. B., Vernon A., Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis 63, e147–e195 (2016). - PMC - PubMed
-
- Carr W., Kurbatova E., Starks A., Goswami N., Allen L., Winston C., Interim Guidance: 4-Month Rifapentine-Moxifloxacin Regimen for the Treatment of Drug-Susceptible Pulmonary Tuberculosis - United States, 2022. MMWR Morb Mortal Wkly Rep 71, 285–289 (2022). - PubMed
-
- World Health Organization, "WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment (https://www.who.int/publications/i/item/9789240048126)," (2022). - PubMed
-
- Fox W., Whither short-course chemotherapy? Br J Dis Chest 75, 331–357 (1981). - PubMed
-
- Campbell J. R., Brode S. K., Barry P., Bastos M. L., Bonnet M., Guglielmetti L., Kempker R., Klimuk D., Laniado Laborin R., Milanov V., Singla R., Skrahina A., Trajman A., van der Werf T. S., Viiklepp P., Menzies D., Association of indicators of extensive disease and rifampin-resistant tuberculosis treatment outcomes: an individual participant data meta-analysis. Thorax 79, 169–178 (2024). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
