Safety and Efficacy of Autologous Bone Marrow Derived Mononuclear Cell Transplant in the Management of Various Neurological Disorders
- PMID: 39803099
- PMCID: PMC11725325
- DOI: 10.7759/cureus.75617
Safety and Efficacy of Autologous Bone Marrow Derived Mononuclear Cell Transplant in the Management of Various Neurological Disorders
Abstract
Background: Cerebral palsy (CP), traumatic spinal cord injury (SCI), and muscular dystrophy (MD), among the various other neurological disorders, are major global health problems because they are chronic disorders with no curative treatments at present. Current interventions aim to relieve symptoms alone and therefore emphasize the necessity for new approaches.
Objective: This study aims to assess the safety and efficacy of autologous bone marrow-derived mononuclear cell (BM-MNC) therapy in patients with CP, traumatic SCI, and MD. Functional improvement and safety are the primary outcomes, while secondary outcomes include patient-reported improvement in quality of life.
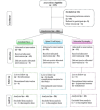
Methods: This was a single-arm, open-label prospective study conducted on 100 patients with CP, SCI, and MD at the GSVM Medical College, Kanpur, India. Bone marrow aspirates were processed via centrifugation, and autologous BM-MNCs were administered intrathecally or intramuscularly. Gross motor function classification system (GMFCS) for CP, the American spinal injury association (ASIA) motor score for SCI, and the north star assessment for limb girdle type dystrophies (NSAD) for MD were used for functional outcome assessment at baseline and at post-treatment cycles. Informed consent was obtained, and the study was approved by the ethics committee. Paired t-tests were used to analyze statistical significance (p<0.05).
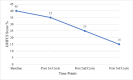
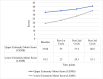
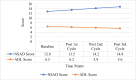
Results: Functional improvements were significant with autologous BM-MNC therapy. Improved motor and cognitive function were shown in CP patients with reduced GMFCS scores (p<0.001). Upper and lower extremity ASIA motor scores improved markedly (p<0.001) in SCI patients. Stabilized muscle strength was seen in MD patients, with increased NSAD and activities of daily living (ADL) scores, suggesting slowing of the disease progression (p<0.019). Side effects were mild and transient.
Conclusion: Autologous BM-MNC therapy appears to be a promising, minimally invasive option for patients with CP, SCI, and MD, which appears to markedly improve functional outcomes and quality of life and may therefore be relevant to clinical practice.
Keywords: bone marrow-derived mononuclear cells; functional improvement; neurological disorders; quality of life; regenerative medicine.
Copyright © 2024, Kala et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Ethical Committee (For Biomedical Health & Research) GSVM Medical College, Kanpur issued approval 106 with Ref No: EC/BMHR/2023/10. The study has also been registered with the Clinical Trials Registry India (CTRI), with the registration details as CTRI/2024/10/074805. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: an open-label phase I trial. Mancías-Guerra C, Marroquín-Escamilla AR, González-Llano O, et al. Cytotherapy. 2014;16:810–820. - PubMed
-
- Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P, Jacob VC. Cell Transplant. 2012;21 Suppl 1:0–90. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
