This is a preprint.
Molecular basis for the assembly of the dynein transport machinery on microtubules
- PMID: 39803430
- PMCID: PMC11722432
- DOI: 10.1101/2024.12.30.630772
Molecular basis for the assembly of the dynein transport machinery on microtubules
Abstract
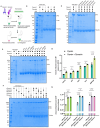
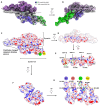
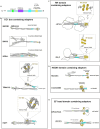
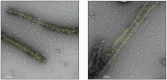
Cytoplasmic dynein-1, a microtubule-based motor protein, requires dynactin and an adaptor to form the processive dynein-dynactin-adaptor (DDA) complex. The role of microtubules in DDA assembly has been elusive. Here, we reveal detailed structural insights into microtubule-mediated DDA assembly using cryo-electron microscopy. We find that an adaptor-independent dynein-dynactin complex (DD) predominantly forms on microtubules in an intrinsic 2:1 stoichiometry, induced by spontaneous parallelization of dynein upon microtubule binding. Adaptors can squeeze in and exchange within the assembled microtubule-bound DD complex, which is enabled by relative rotations between dynein and dynactin, and further facilitated by dynein light intermediate chains that assist in an adaptor 'search' mechanism. Our findings elucidate the dynamic adaptability of the dynein transport machinery, and reveal a new mode for assembly of the motile complex.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures














References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
