This is a preprint.
Neuronal LAG3 facilitates pathogenic α-synuclein neuron-to-neuron propagation
- PMID: 39803449
- PMCID: PMC11722393
- DOI: 10.1101/2025.01.03.631221
Neuronal LAG3 facilitates pathogenic α-synuclein neuron-to-neuron propagation
Abstract
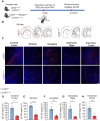
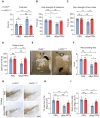
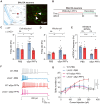
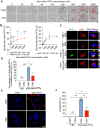
Lymphocyte activation gene 3 (LAG3) is a key receptor involved in the propagation of pathological proteins in Parkinson's disease (PD). This study investigates the role of neuronal LAG3 in mediating the binding, uptake, and propagation of α-synuclein (αSyn) preformed fibrils (PFFs). Using neuronal LAG3 conditional knockout mice and human induced pluripotent stem cells-derived dopaminergic (DA) neurons, we demonstrate that LAG3 expression is critical for pathogenic αSyn propagation. Our results show that the absence of neuronal LAG3 significantly reduces αSyn pathology, alleviates motor dysfunction, and inhibits neurodegeneration in vivo. Electrophysiological recordings revealed that αSyn PFFs induce pronounced neuronal hyperactivity in wild-type (WT) neurons, increasing firing rates in cell-attached and whole-cell configurations, and reducing miniature excitatory postsynaptic currents. In contrast, neurons lacking LAG3 resisted these electrophysiological effects. Moreover, treatment with an anti-human LAG3 antibody in human DA neurons inhibited αSyn PFFs binding and uptake, preventing pathology propagation. These findings confirm the essential function of neuronal LAG3 in mediating αSyn propagation and associated disruptions, identifying LAG3 as a potential therapeutic target for PD and related α-synucleinopathies.
Keywords: LAG3; PFFs; electrophysiology; neuron; α-synuclein.
Conflict of interest statement
Competing interests: TMD, VLD and XM has filed a patent for therapeutic uses of LAG3 (application No: PCT/US2017/047878). DAAV and CJW are inventors on issued patents (US [8,551,481]; Europe [1897548]; Australia [2004217526], Hong Kong [1114339]) held by St Jude Children’s Research Hospital and Johns Hopkins University that cover LAG3. Additional applications pending. DAAV: cofounder and stock holder – Novasenta, Potenza, Tizona, Trishula; stock holder – Werewolf; patents licensed and royalties - BMS, Novasenta; scientific advisory board member - Werewolf, F-Star, Apeximmune, T7/Imreg Bio; consultant - BMS, Regeneron, Ono Pharma, Avidity Partners, Peptone, Third Arc Bio, Secarna, Curio Bio; funding - BMS, Novasenta. All other authors declare no conflicts of interest.
Figures
References
-
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. Epub 2011/10/11. doi: 10.1016/j.neuron.2011.08.033. - DOI - PMC - PubMed
-
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A. 2013;110(33):E3138–47. Epub 20130729. doi: 10.1073/pnas.1301440110. - DOI - PMC - PubMed
Publication types
Grants and funding
- P30 NS050274/NS/NINDS NIH HHS/United States
- R01 AG073291/AG/NIA NIH HHS/United States
- R01 AG071820/AG/NIA NIH HHS/United States
- R01 AI144422/AI/NIAID NIH HHS/United States
- R01 NS107318/NS/NINDS NIH HHS/United States
- R01 AG085688/AG/NIA NIH HHS/United States
- R21 NS125559/NS/NINDS NIH HHS/United States
- RF1 AG079487/AG/NIA NIH HHS/United States
- K01 AG056841/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- R01 AG089605/AG/NIA NIH HHS/United States
- RF1 NS137428/NS/NINDS NIH HHS/United States
- RF1 NS125592/NS/NINDS NIH HHS/United States
- P01 AI108545/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
