This is a preprint.
Extracellular vesicles from diverse fungal pathogens induce species-specific and endocytosis-dependent immunomodulation
- PMID: 39803513
- PMCID: PMC11722428
- DOI: 10.1101/2025.01.03.631181
Extracellular vesicles from diverse fungal pathogens induce species-specific and endocytosis-dependent immunomodulation
Update in
-
Extracellular vesicles from diverse fungal pathogens induce species-specific and endocytosis-dependent immunomodulation.PLoS Pathog. 2025 May 30;21(5):e1012879. doi: 10.1371/journal.ppat.1012879. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40445992 Free PMC article.
Abstract
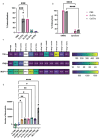
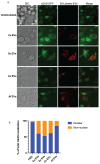
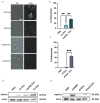
Microbial pathogens generate extracellular vesicles (EVs) for intercellular communication and quorum sensing. Microbial EVs also induce inflammatory pathways within host innate immune cells. We previously demonstrated that EVs secreted by Candida albicans trigger type I interferon signaling in host cells specifically via the cGAS-STING innate immune signaling pathway. Here, we show that despite sharing similar properties of morphology and internal DNA content, the interactions between EVs and the innate immune system differ according to the parental fungal species. EVs secreted by C. albicans, Saccharomyces cerevisiae, Cryptococcus neoformans, and Aspergillus fumigatus are endocytosed at different rates by murine macrophages triggering varied cytokine responses, innate immune signaling, and subsequent immune cell recruitment. Notably, cell wall constituents that decorate C. neoformans and A. fumigatus EVs inhibit efficient internalization by macrophages and dampen innate immune activation. Our data uncover the transcriptional and functional consequences of the internalization of diverse fungal EVs by immune cells and reveal novel insights into the early innate immune response to distinct clinically significant fungal pathogens.
Figures


References
-
- Pekmezovic M, Hovhannisyan H, Gresnigt MS, Iracane E, Oliveira-Pacheco J, Siscar-Lewin S, et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat Microbiol. 2021. May 1;6(5):643–57. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
