This is a preprint.
The C. elegans homolog of Sjögren's Syndrome Nuclear Antigen 1 is required for the structural integrity of the centriole and bipolar mitotic spindle assembly
- PMID: 39803516
- PMCID: PMC11722412
- DOI: 10.1101/2024.10.03.616528
The C. elegans homolog of Sjögren's Syndrome Nuclear Antigen 1 is required for the structural integrity of the centriole and bipolar mitotic spindle assembly
Update in
-
C. elegans SSNA-1 is required for the structural integrity of centrioles and bipolar spindle assembly.Nat Commun. 2025 Jun 5;16(1):5220. doi: 10.1038/s41467-025-59939-0. Nat Commun. 2025. PMID: 40473601 Free PMC article.
Abstract
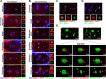
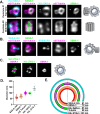
Centrioles play central roles in ciliogenesis and mitotic spindle assembly. Once assembled, centrioles exhibit long-term stability, a property essential for maintaining numerical control. How centriole stability is achieved and how it is lost in certain biological contexts are still not completely understood. In this study we show that SSNA-1, the Caenorhabditis elegans ortholog of Sjogren's Syndrome Nuclear Antigen 1, is a centriole constituent that localizes close to the microtubule outer wall, while also exhibiting a developmentally regulated association with centriole satellite-like structures. A complete deletion of the ssna-1 gene results in an embryonic lethal phenotype marked by the appearance of extra centrioles and spindle poles. We show that SSNA-1 genetically interacts with the centriole stability factor SAS-1 and is required post assembly for centriole structural integrity. In SSNA-1's absence, centrioles assemble but fracture leading to extra spindle poles. However, if the efficiency of cartwheel assembly is reduced, the absence of SSNA-1 results in daughter centriole loss and monopolar spindle formation, indicating that the cartwheel and SSNA-1 cooperate to stabilize the centriole during assembly. Our work thus shows that SSNA-1 contributes to centriole stability during and after assembly, thereby ensuring proper centriole number.
Figures





References
-
- Hilbert M., Noga A., Frey D., Hamel V., Guichard P., Kraatz S.H., Pfreundschuh M., Hosner S., Fluckiger I., Jaussi R., et al. (2016). SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture. Nat Cell Biol 18, 393–403. 10.1038/ncb3329. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
