This is a preprint.
Convergent state-control of endogenous opioid analgesia
- PMID: 39803541
- PMCID: PMC11722426
- DOI: 10.1101/2025.01.03.631111
Convergent state-control of endogenous opioid analgesia
Abstract
Pain is a dynamic and nonlinear experience shaped by injury and contextual factors, including expectations of future pain or relief1. While μ opioid receptors are central to the analgesic effects of opioid drugs, the endogenous opioid neurocircuitry underlying pain and placebo analgesia remains poorly understood. The ventrolateral column of the posterior periaqueductal gray is a critical hub for nociception and endogenous analgesia mediated by opioid signaling2. However, significant gaps remain in understanding the cell-type identities, the sub-second neural dynamics involved in pain modulation, the role of endogenous peptide neuromodulators, and the contextual factors influencing these processes. Using spatial mapping with single-nuclei RNA sequencing of pain-active neurons projecting to distinct long-range brain targets, alongside cell type-specific and activity-dependent genetic tools for in vivo optical recordings and modulation of neural activity and opioid peptide release, we identified a functional dichotomy in the ventrolateral periaqueductal gray. Neurons expressing μ opioid receptors encode active nociceptive states, whereas enkephalin-releasing neurons drive pain relief during recovery from injury, in response to learned fear predictions, and during placebo analgesia. Finally, by leveraging the functional effects of placebo analgesia, we used direct optogenetic activation of vlPAG enkephalin neurons to drive opioid peptide release, resulting in a robust reduction in pain. These findings show that diverse need states converge on a shared midbrain circuit that releases endogenous opioids with high spatiotemporal precision to suppress nociceptive activity and promote analgesia.
Conflict of interest statement
COMPETING INTERESTS G.C, K.D., C.R. are inventors on a provisional patent application through the University of Pennsylvania and Stanford University regarding the custom sequences used to develop, and the applications of mMORp and hMORp constructs (patent application number: 63/383,462 462 ‘Human and Murine Oprm1 Promoters and Uses Thereof’). B.C.R. receives research funding from Novo Nordisk and Boehringer Ingelheim and in-kind support from 10x Genomics and Oxford Nanopore Technologies that were not used in support of these studies.
Figures

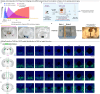





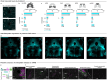












References
-
- Carrive P. & Morgan M. M. Periaqueductal Gray. The Human Nervous System: Second Edition 393–423 (2004) doi: 10.1016/B978-012547626-3/50013-2. - DOI
Publication types
Grants and funding
- R21 DA055846/DA/NIDA NIH HHS/United States
- F31 NS125927/NS/NINDS NIH HHS/United States
- F32 DA055458/DA/NIDA NIH HHS/United States
- R00 DA043609/DA/NIDA NIH HHS/United States
- DP2 GM140923/GM/NIGMS NIH HHS/United States
- R01 NS130044/NS/NINDS NIH HHS/United States
- F99 NS135765/NS/NINDS NIH HHS/United States
- R01 DA056829/DA/NIDA NIH HHS/United States
- F32 DA053099/DA/NIDA NIH HHS/United States
- R01 DK139386/DK/NIDDK NIH HHS/United States
- U01 NS113295/NS/NINDS NIH HHS/United States
- R01 DA056599/DA/NIDA NIH HHS/United States
- R01 DA054374/DA/NIDA NIH HHS/United States
- U01 NS120820/NS/NINDS NIH HHS/United States
- R01 DK128475/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
