Phenylbutyric Acid Modulates Apoptosis and ER Stress-Related Gene Expression in Glycogen Storage Disease Type Ib In Vitro Model
- PMID: 39803753
- PMCID: PMC11726116
- DOI: 10.1002/mgg3.70054
Phenylbutyric Acid Modulates Apoptosis and ER Stress-Related Gene Expression in Glycogen Storage Disease Type Ib In Vitro Model
Abstract
Introduction: Chronic endoplasmic reticulum (ER) stress and increased apoptosis are involved in the pathogenesis of glycogen storage disease Ib (GSD Ib), whereas small molecule phenylbutyrate (4-PBA) showed the capability of reducing ER stress-induced apoptosis. The objective was to generate an in vitro system in which capability of small molecules (SMs) to influence ER stress and apoptosis could be screened at the expression level.
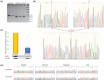
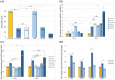
Methods: G6PT-deficient FlpInHEK293 cell line was created and validated using the CRISPR/Cas9 knockout method. Molecular markers of unfolded protein response (ATF4, DDIT3, HSPA5, XBP1s), and apoptosis (BCL2/BAX, CASP3, CASP7) in G6PT-deficient cells were analyzed using RT-qPCR method before and upon the treatment with 4-PBA.
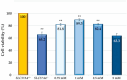
Results: Treatment with the most effective dose of 1 mM 4-PBA reduced the expression of UPR markers and executioner caspases, while increased BCL2/BAX ratio in G6PT-deficient cells. Our results proved the concept that 4-PBA could alleviate markers of ER stress detected in the GSD Ib in vitro model system and prevent cell death.
Conclusion: This cost-effective in vitro model screens the therapeutic potential of SMs affecting ER stress and apoptosis in G6PT-deficient kidney cells, offering a first-line screening assay for promising compounds. 4-PBA's potential repurposing for GSD Ib patients opens new research directions.
Keywords: 4‐PBA; CRISPR/Cas9; ER stress; GSD Ib in vitro model system; apoptosis.
© 2025 The Author(s). Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Adachi, T. , Yasuda H., Nakamura S., et al. 2011. “Endoplasmic Reticulum Stress Induces Retinal Endothelial Permeability of Extracellular‐Superoxide Dismutase.” Free Radical Research 45, no. 9: 1083–1092. - PubMed
-
- Andjelkovic, M. , Skakic A., Ugrin M., et al. 2022. “Crosstalk Between Glycogen‐Selective Autophagy, Autophagy and Apoptosis as a Road Towards Modifier Gene Discovery and New Therapeutic Strategies for Glycogen Storage Diseases.” Life (Basel) 12, no. 9: 12091396. 10.3390/life12091396. - DOI - PMC - PubMed
-
- Bhattarai, K. R. , Chaudhary M., Kim H. R., and Chae H. J.. 2020. “Endoplasmic Reticulum (ER) Stress Response Failure in Diseases.” Trends in Cell Biology 30, no. 9: 672–675. - PubMed
-
- Cao, S. S. , and Kaufman R. J.. 2013. “Targeting Endoplasmic Reticulum Stress in Metabolic Disease.” Expert Opinion on Therapeutic Targets 17, no. 4: 437–448. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

