Anti-inflammatory effect of Angiotensin 1-7 in white adipose tissue
- PMID: 39803918
- PMCID: PMC11730366
- DOI: 10.1080/21623945.2024.2449027
Anti-inflammatory effect of Angiotensin 1-7 in white adipose tissue
Abstract
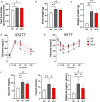
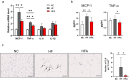
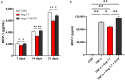
Obesity is a global health concern that promotes chronic low-grade inflammation, leading to insulin resistance, a key factor in many metabolic diseases. Angiotensin 1-7 (Ang 1-7), a component of the renin-angiotensin system (RAS), exhibits anti-inflammatory effects in obesity and related disorders, though its mechanisms remain unclear. In this study, we examined the effect of Ang 1-7 on inflammation of white adipose tissue (WAT) in dietary-induced obese mice. Monocyte chemoattractant protein-1 (MCP-1) produced by white adipocytes and tumour necrosis factor-α (TNF-α) produced by macrophages are pro-inflammatory cytokines and interact to form a pathogenic loop to exacerbate obesity-induced inflammation. We found that Ang 1-7 reduced MCP-1 and TNF-α gene expressions and the number of crown-like structures, which are histological hallmarks of the pro-inflammatory process, in visceral epididymal WAT (eWAT) and reduced circulating MCP-1 and TNF-α levels, accompanied by improvement in insulin resistance, in dietary-induced obese mice. Furthermore, Ang 1-7 reduced MCP-1 and TNF-α secretions in 3T3-L1 white adipocytes and RAW 264.7 macrophages, respectively, which are in vitro experimental models mimicking obesity condition. Our results suggest that Ang 1-7 directly acts on WAT to mitigate obesity-induced inflammation. Thus, this study provides novel insights into the underlying mechanism of anti-obesity effects of Ang 1-7.
Keywords: Angiotensin 1-7; MCP-1; TNF-α; anti-inflammatory effect; obesity.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
