TAK-925 (Danavorexton), an Orexin Receptor 2 Agonist, Reduces Opioid-induced Respiratory Depression and Sedation without Affecting Analgesia in Healthy Men
- PMID: 39804333
- PMCID: PMC11892998
- DOI: 10.1097/ALN.0000000000005375
TAK-925 (Danavorexton), an Orexin Receptor 2 Agonist, Reduces Opioid-induced Respiratory Depression and Sedation without Affecting Analgesia in Healthy Men
Abstract
Background: Orexin neuropeptides help regulate sleep/wake states, respiration, and pain. However, their potential role in regulating breathing, particularly in perioperative settings, is not well understood. TAK-925 (danavorexton), a novel orexin receptor 2-selective agonist, directly activates neurons associated with respiratory control in the brain and improves respiratory parameters in rodents undergoing fentanyl-induced sedation. This study assessed the safety and effect of danavorexton on ventilation in healthy men in an established remifentanil-induced respiratory depression model.
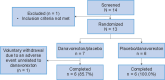
Methods: This single-center, double-blind, placebo-controlled, two-way crossover, phase 1 trial randomized (1:1) 13 healthy men to danavorexton (11 mg [low-dose], then 19 mg [high-dose]) or placebo, under remifentanil infusion, on two occasions separated by a 36-h or longer washout period. Remifentanil infusion was titrated under isohypercapnic conditions to achieve an approximately 30 to 40% decrease in minute ventilation (from approximately 20 to approximately 14 l/min) before danavorexton/placebo administration. Assessments included safety, ventilation measurements, sedation, and pain tolerance.
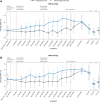
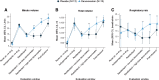
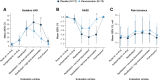
Results: A total of four (30.8%) danavorexton-treated participants and one (8.3%) placebo-treated participant experienced treatment-emergent adverse events (all mild in severity). Insomnia, lasting 1 day, occurred in one participant, and was considered related to danavorexton. Compared with placebo, low- and high-dose danavorexton significantly increased ventilation variables (observed mean [95% CI] change, sensitivity analysis model-based P values) including minute volume (8.2 [95% CI, 5.0 to 11.4] and 13.0 [95% CI, 9.4 to 16.5] l/min), tidal volume (312 [95% CI, 180 to 443] and 483 [95% CI, 309 to 657] ml), and respiratory rate (3.8 [95% CI, 1.9 to 5.7] and 5.2 [95% CI, 2.7 to 7.7] breaths/min; all P < 0.001). High-dose danavorexton significantly decreased sedation on a visual analog scale (-29.7 [95% CI, -54.1 to -5.3] mm; P < 0.001) and the Richmond Agitation Sedation Scale (0.4 [95% CI, 0.0 to 0.7]; P < 0.001) compared with placebo. Improvements in respiratory variables continued beyond completion of danavorexton infusion. No significant differences in pain tolerance were observed between danavorexton doses or between danavorexton and placebo (approximately 13% increase from baseline; low dose, P = 0.491; high dose, P = 0.140).
Conclusions: Danavorexton has effects on respiration and wakefulness in an opioid-induced respiratory depression setting without reversing opioid analgesia.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc., on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
Dr. Dahan received consultancy fees from Enalare Therapeutics Inc. (Princeton, New Jersey), Trevena Inc. (Chesterbrook, Pennsylvania), and Takeda Pharmaceuticals International AG (Zurich, Switzerland). Drs. Hang, Lu, Naylor, Olsson, Sheikh, Sullivan, Tolkoff, von Rosenstiel, Wu, and Meyer are employees of Takeda Development Center Americas, Inc. (Lexington, Massachusetts) and stockholders of Takeda Pharmaceutical Company, Ltd. (Osaka, Japan). The other authors declare no competing interests.
The article processing charge was funded by Takeda Development Center Americas, Inc., Lexington, Massachusetts.
Figures

References
-
- Dahan A, Niesters M, Smith T, Overdyk F: Opioids. Clinical Anesthesia, 8th edition. Edited by Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, Ortega R, Sharar SR, Holt NF. New York, Wolters Kluwer, 2017, pp 505–26
-
- Khanna AK, Bergese SD, Jungquist CR, et al. ; PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY Group Collaborators: Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: An international prospective, observational trial. Anesth Analg 2020; 131:1012–24 - PMC - PubMed
-
- Algera MH, Kamp J, van der Schrier R, et al. : Opioid-induced respiratory depression in humans: A review of pharmacokinetic-pharmacodynamic modelling of reversal. Br J Anaesth 2019; 122:e168–79 - PubMed
-
- Baldo BA, Rose MA: Mechanisms of opioid-induced respiratory depression. Arch Toxicol 2022; 96:2247–60 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

