Trifluridine/Tipiracil (FTD/TPI) in Metastatic Colorectal Cancer in Hong Kong: A Territory-Wide Cohort Study
- PMID: 39804541
- PMCID: PMC11787203
- DOI: 10.1007/s12325-024-03077-4
Trifluridine/Tipiracil (FTD/TPI) in Metastatic Colorectal Cancer in Hong Kong: A Territory-Wide Cohort Study
Abstract
Introduction: Randomized phase III trials showed that using trifluridine/tipiracil (FTD/TPI) in patients with pre-treated metastatic colorectal cancer (mCRC) conferred survival benefit versus placebo. Here, we investigated the effectiveness and safety of FTD/TPI and sought to identify prognostic factors among the mCRC population in Hong Kong.
Methods: A non-interventional, retrospective, multicenter cohort study enrolled patients with mCRC who received FTD/TPI in seven public hospitals in Hong Kong between 2016 and 2020. Overall survival (OS) was the primary endpoint; treatment duration and occurrence of neutropenia were secondary endpoints. We also performed a post hoc analysis to identify factors influencing OS and treatment duration.
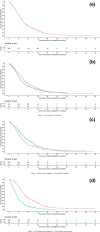
Results: Overall, 456 patients were included (median age, 64.0 years; 57.5% men). Approximately half (225/456; 49.3%) had RAS wild-type tumors; the median treatment duration was 12.4 weeks (95% confidence interval [CI] 11.1-13.1). Median OS was 7.59 months (95% CI 7.00-8.21). Overall, 289 (63.4%) patients developed neutropenia of any grade and 159 (34.9%) developed grade ≥ 3 neutropenia. Neutropenia at 1 month occurred in 193 (43.1%) patients. The use of granulocyte colony-stimulating factor for neutropenia was reported for 42 (9.2%) patients. The development of neutropenia, absolute neutrophil count decrease of ≥ 2 grades in 1 month, absence of liver metastasis, and RAS wild-type status were associated with significantly longer OS and, except for RAS wild-type status (not analyzed), longer treatment duration (p < 0.05 for all comparisons).
Conclusion: Our data show that treatment with FTD/TPI offers survival benefits in patients with refractory mCRC in Hong Kong consistent with randomized controlled trials and other real-world studies. Furthermore, the prognosis in patients receiving FTD/TPI appears to be significantly better in those who develop neutropenia, with RAS wild-type status, or those without liver metastases, despite a higher rate of dose reduction in the real-world setting.
Keywords: FTD/TPI; Metastatic colorectal cancer; Neutropenia; Prognostic factor; Real-world; TAS-102; Trifluridine/tipiracil.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Karen Hoi-Lam Li has received support for meeting attendance and travel from Taiho Pharma Asia Pacific Pte. Ltd. Ka-On Lam has received speaking honoraria from Amgen Inc., Astellas Pharma Inc., AstraZeneca PLC, Bayer AG, Bristol Myers Squibb, Daiichi Sankyo Co. Ltd., Eli Lilly and Co., GSK PLC, Merck & Co. Inc., Novartis AG, F. Hoffmann-La Roche Ltd., Sanofi, and Taiho Pharma Asia Pacific Pte. Ltd. Ka-On Lam has also received meeting and travel support from Merck & Co. Inc. and Taiho Pharma Asia Pacific Pte. Ltd. and advisory board honoraria from Amgen Inc., Astellas Pharma Inc., and GSK PLC. Roland Ching-Yu Leung, Vikki Tang and Thomas Chung-Cheung Yau have no conflicts of interest to disclose. No payment or honoraria have been received by any author for this article. Ethical Approval: The study protocol was reviewed for ethics, science and compliance with the Declaration of Helsinki, ICH GCP guidelines, local regulations, Hospital Authority and university policies. The protocol, encompassing the use of CDARS data from all participating centers, was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster before any data collection occurred (IRB reference number UW 19-460). The requirement for informed consent was waived owing to the anonymous nature of the data.
Figures
Similar articles
-
Prognostic impact and risk factors of severe neutropenia in the early phase of treatment with trifluridine-tipiracil for metastatic colorectal cancer patients: a single-center retrospective study.Int J Colorectal Dis. 2025 Jan 13;40(1):11. doi: 10.1007/s00384-024-04798-2. Int J Colorectal Dis. 2025. PMID: 39800823 Free PMC article.
-
Real-World Adherence in Patients with Metastatic Colorectal Cancer Treated with Trifluridine plus Tipiracil or Regorafenib.Oncologist. 2020 Jan;25(1):e75-e84. doi: 10.1634/theoncologist.2019-0240. Epub 2019 Oct 7. Oncologist. 2020. PMID: 31591140 Free PMC article.
-
Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: a retrospective study.Cancer Chemother Pharmacol. 2020 Sep;86(3):427-433. doi: 10.1007/s00280-020-04129-6. Epub 2020 Aug 20. Cancer Chemother Pharmacol. 2020. PMID: 32816155
-
Efficacy and Safety of Trifluridine/Tipiracil-Containing Combinations in Colorectal Cancer and Other Advanced Solid Tumors: A Systematic Review.Oncologist. 2024 May 3;29(5):e601-e615. doi: 10.1093/oncolo/oyae007. Oncologist. 2024. PMID: 38366864 Free PMC article.
-
A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer.Acta Oncol. 2019 Aug;58(8):1149-1157. doi: 10.1080/0284186X.2019.1605192. Epub 2019 Apr 19. Acta Oncol. 2019. PMID: 31002008
References
-
- Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Curr Gastroenterol Rep. 2019;21:36. - PubMed
-
- World Health Organization. Colorectal Cancer: Globocan. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact.... Accessed 15 Feb 2022.
-
- Hong Kong Cancer Registry. 10 most common cancers in Hong Kong in 2019. https://www3.ha.org.hk/cancereg/. Accessed 6 May 2023.
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Colon and Rectum Cancer. https://seer.cancer.gov/explorer/application.html. Accessed 16 Mar 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

