Five-Year Results With Patisiran for Hereditary Transthyretin Amyloidosis With Polyneuropathy: A Randomized Clinical Trial With Open-Label Extension
- PMID: 39804640
- PMCID: PMC11894486
- DOI: 10.1001/jamaneurol.2024.4631
Five-Year Results With Patisiran for Hereditary Transthyretin Amyloidosis With Polyneuropathy: A Randomized Clinical Trial With Open-Label Extension
Abstract
Importance: There is a lack of long-term efficacy and safety data on hereditary transthyretin amyloidosis with polyneuropathy (hATTR-PN) and on RNA interference (RNAi) therapeutics in general. This study presents the longest-term data to date on patisiran for hATTR-PN.
Objective: To present the long-term efficacy and safety of patisiran in adults with hATTR-PN.
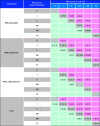
Design, setting, and participants: This global open-label extension (OLE) of the APOLLO randomized clinical trial and phase 2 OLE study enrolled patients from 43 hospitals or clinical centers across 19 countries between July 2015 and August 2017, with follow-up until November 2022. Of 212 eligible patients with hATTR who completed the phase 3 APOLLO or phase 2 OLE parent studies, 211 enrolled in and 138 completed the global OLE.
Intervention: Patisiran, 0.3 mg/kg, intravenously once every 3 weeks for up to 5 years.
Main outcomes and measures: Outcomes evaluated at year 5 of the global OLE included disability (polyneuropathy disability [PND] score); polyneuropathy severity (Neuropathy Impairment Score [NIS]), nutritional status (modified body mass index [mBMI]), quality of life (Norfolk Quality of Life-Diabetic Neuropathy [Norfolk QOL-DN]), and Rasch-Built Overall Disability Scale (R-ODS), with no statistical hierarchy. Safety, survival probability, and mortality were also assessed.
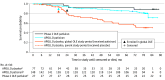
Results: At the global OLE baseline, the mean (SD) age was 61.3 (12.3) years, and 156 patients (73.9%) were male. In 138 patients completing the study, PND scores remained stable or improved in 89 patients (65.0%), NISs showed a mean (SD) change of 10.9 (14.7), and mean (SD) mBMI (calculated as weight in kilograms divided by height in meters squared times serum albumin in grams per liter) increased by 46.4 (120.7) over 5 years from baseline. Norfolk QOL-DN and R-ODS scores showed mean (SD) changes of 4.1 (16.7) and -3.7 (6.2), respectively. Adverse events (AEs) leading to study withdrawal occurred in 47 patients (22.3%). Infusion-related reactions were the most common treatment-related AE (n = 34 [16.1%]). Overall, 41 patients (19.4%) died during the study. Patisiran treatment in the parent study and low familial amyloid polyneuropathy score at parent study baseline were associated with significantly improved survival.
Conclusions and relevance: In the longest study of an RNAi therapeutic for any disease, patisiran treatment resulted in modest changes for patients with hATTR-PN with an acceptable safety profile. These results highlight the importance of initiating early treatment for hATTR and the potential of RNAi therapeutics in medicine.
Trial registration: ClinicalTrials.gov Identifier: NCT02510261.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

