Androgen Receptor Pathway Inhibitor Therapy for Advanced Prostate Cancer: Secondary Analysis of a Randomized Clinical Trial
- PMID: 39804646
- PMCID: PMC11731179
- DOI: 10.1001/jamanetworkopen.2024.54253
Androgen Receptor Pathway Inhibitor Therapy for Advanced Prostate Cancer: Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: The open-label randomized phase 2 LACOG0415 trial evaluated 3 treatment strategies for patients with advanced castration-sensitive prostate cancer (CSPC): androgen deprivation therapy (ADT) plus abiraterone acetate and prednisone (AAP), apalutamide (APA) alone, or APA plus AAP.
Objective: To investigate the association of ADT plus AAP, APA alone, or APA plus AAP with health-related quality of life (HRQOL) in patients with advanced CSPC in the LACOG0415 trial.
Design, setting, and participants: The LACOG0415 randomized clinical trial comprised 128 patients with advanced CSPC who were randomized (1:1:1) to 1 of 3 treatment arms from October 16, 2017, to April 23, 2019. Statistical analysis was conducted from March to September 2022.
Interventions: Patients were randomized (1:1:1) to 1 of 3 treatment arms: ADT plus AAP, APA alone, or APA plus AAP.
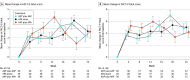
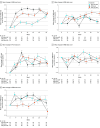
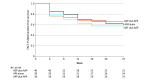
Main outcomes and measures: Health-related quality of life was evaluated using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire, including its subscales, completed at baseline and every 4 weeks until week 25. FACT-P scores range from 0 to 156, and higher scores indicate better HRQOL. Mean changes in score from baseline to week 25 were adjusted by baseline score and were calculated to evaluate whether there was a difference according to the treatment arm using a mixed-effect model for repeated measures. Time to deterioration was estimated by Kaplan-Meier curves and compared by stratified log-rank test. Analysis was performed on an intention-to-treat basis.
Results: A total of 128 patients with advanced CSPC were randomized to receive ADT plus AAP (n = 42; median age, 69.8 years [IQR, 58.9-71.6 years]), APA alone (n = 42; median age, 69.5 years [IQR, 59.8-72.6 years]), or APA plus AAP (n = 44; median age, 71.0 years [IQR, 63.0-72.3 years]). Metastatic disease was present in 95 patients (74.2%), high-risk biochemical recurrence disease in 22 (17.2%), and locally advanced disease in 11 (8.6%). There was no significant difference in baseline mean (SD) FACT-P total scores and subscales among the 3 treatment arms (FACT-P total score: ADT plus AAP arm, 118.5 [24.3]; APA alone arm, 116.1 [23.9]; AAP plus APA arm, 114.9 [18.1]; P = .69). Health-related quality of life was maintained during treatment period, and there were no statistically significant differences at 25 weeks in mean (SD) FACT-P total scores or subscales between treatment arms (FACT-P total score: ADT plus AAP arm, 122.3 [20.4]; APA alone arm, 119.5 [16.4]; AAP plus APA arm, 119.9 [20.3]). The APA alone and AAP plus APA arms were not associated with meaningful improvements in HRQOL compared with the ADT plus AAP arm, except in time to deterioration of the emotional well-being score, which was more favorable in the APA alone arm (reference arm: ADT plus AAP arm; APA alone arm: hazard ratio, 0.37 [0.15-0.85]; P = .02; ADT plus AAP arm: hazard ratio, 0.56 [0.26-1.19]; P = .13). Limitations include short follow-up period and the absence of other questionnaires to capture differences between therapies.
Conclusions and relevance: In this prespecified secondary analysis of a randomized clinical trial of ADT plus AAP, APA alone, or APA plus AAP for patients with advanced CSPC, HRQOL was not statistically different between treatments with APA alone or APA plus AAP as compared with ADT plus AAP. Larger studies with longer follow-up and more specific questionnaires are needed to further evaluate HRQOL with these treatment strategies.
Trial registration: ClinicalTrials.gov Identifier: NCT02867020.
Conflict of interest statement
Figures
References
-
- Davis ID, Martin AJ, Stockler MR, et al. ; ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. doi:10.1056/NEJMoa1903835 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

