Intra-Arterial Tenecteplase Following Endovascular Reperfusion for Large Vessel Occlusion Acute Ischemic Stroke: The POST-TNK Randomized Clinical Trial
- PMID: 39804681
- PMCID: PMC11836761
- DOI: 10.1001/jama.2024.23466
Intra-Arterial Tenecteplase Following Endovascular Reperfusion for Large Vessel Occlusion Acute Ischemic Stroke: The POST-TNK Randomized Clinical Trial
Abstract
Importance: The impact of adjunctive intra-arterial tenecteplase administration following near-complete to complete reperfusion by endovascular thrombectomy (EVT) for acute ischemic stroke is unknown.
Objective: To assess the efficacy and adverse events of adjunctive intra-arterial tenecteplase in patients with large vessel occlusion stroke who had achieved near-complete to complete reperfusion (defined as a score on the expanded Thrombolysis in Cerebral Infarction [eTICI] scale of 2c to 3) after EVT.
Design, setting, and participants: Investigator-initiated, randomized, open-label, blinded outcome assessment trial implemented at 34 hospitals in China among 540 patients with stroke due to proximal intracranial large vessel occlusion within 24 hours of the time they were last known to be well, with an eTICI score of 2c to 3 after EVT, and without prior intravenous thrombolysis. Recruitment took place between October 26, 2022, and March 1, 2024, with final follow-up on June 3, 2024.
Interventions: Eligible patients were randomly assigned to receive intra-arterial tenecteplase (n = 269) at 0.0625 mg/kg or no intra-arterial thrombolysis (control group; n = 271).
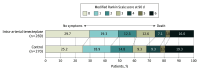
Main outcomes and measures: The primary efficacy outcome was freedom from disability, defined as a score of 0 or 1 on the modified Rankin Scale (range, 0 [no symptoms] to 6 [death]) at 90 days. The primary safety outcomes were death at 90 days and symptomatic intracranial hemorrhage within 48 hours.
Results: A total of 539 participants (99.8%) completed the trial (median age, 69 years; 221 female [40.9%]). The proportion with a modified Rankin Scale score of 0 or 1 at 90 days was 49.1% (132/269) in the intra-arterial tenecteplase group and 44.1% (119/270) in the control group (adjusted risk ratio, 1.15 [95% CI, 0.97-1.36]; P = .11). Ninety-day mortality was 16.0% and 19.3% (adjusted hazard ratio, 0.75 [95% CI, 0.50-1.13]; P = .16), respectively. The proportions of symptomatic intracranial hemorrhage were 6.3% and 4.4% (adjusted risk ratio, 1.43 [95% CI, 0.68-2.99]; P = .35), respectively.
Conclusions and relevance: In patients with acute ischemic stroke due to large vessel occlusion presenting within 24 hours of time last known to be well and who had achieved near-complete to complete reperfusion after EVT, adjunctive intra-arterial tenecteplase did not significantly increase the likelihood of freedom from disability at 90 days.
Trial registration: ChiCTR.org.cn Identifier: ChiCTR2200064809.
Conflict of interest statement
Figures


Comment in
-
Intra-Arterial Thrombolytics During Thrombectomy for Ischemic Stroke-End of the Story or a New Beginning?JAMA. 2025 Feb 18;333(7):571-573. doi: 10.1001/jama.2024.27100. JAMA. 2025. PMID: 39804672 No abstract available.
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 - DOI - PubMed
-
- Majoie CB, Cavalcante F, Gralla J, et al. ; IRIS Collaborators . Value of intravenous thrombolysis in endovascular treatment for large-vessel anterior circulation stroke: individual participant data meta-analysis of six randomised trials. Lancet. 2023;402(10406):965-974. doi: 10.1016/S0140-6736(23)01142-X - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

