ADAMTS4-Specific MR Peptide Probe for the Assessment of Atherosclerotic Plaque Burden in a Mouse Model
- PMID: 39804796
- PMCID: PMC12233170
- DOI: 10.1097/RLI.0000000000001152
ADAMTS4-Specific MR Peptide Probe for the Assessment of Atherosclerotic Plaque Burden in a Mouse Model
Abstract
Introduction: Atherosclerosis is the underlying cause of multiple cardiovascular pathologies. The present-day clinical imaging modalities do not offer sufficient information on plaque composition or rupture risk. A disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4) is a strongly upregulated proteoglycan-cleaving enzyme that is specific to cardiovascular diseases, inter alia, atherosclerosis.
Materials and methods: Male apolipoprotein E-deficient mice received a high-fat diet for 2 (n = 11) or 4 months (n = 11). Additionally, a group (n = 11) receiving pravastatin by drinking water for 4 months alongside the high-fat diet was examined. The control group (n = 10) consisted of C57BL/6J mice on standard chow. Molecular magnetic resonance imaging was performed prior to and after administration of the gadolinium (Gd)-based ADAMTS4-specific probe, followed by ex vivo analyses of the aortic arch, brachiocephalic arteries, and carotid arteries. A P value <0.05 was considered to indicate a statistically significant difference.
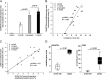
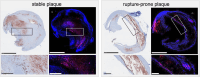
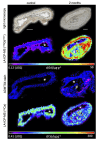
Results: With advancing atherosclerosis, a significant increase in the contrast-to-noise ratio was measured after intravenous application of the probe (mean precontrast = 2.25; mean postcontrast = 11.47, P < 0.001 in the 4-month group). The pravastatin group presented decreased ADAMTS4 expression. A strong correlation between ADAMTS4 content measured via immunofluorescence staining and an increase in the contrast-to-noise ratio was detected ( R2 = 0.69). Microdissection analysis revealed that ADAMTS4 gene expression in the plaque area was significantly greater than that in the arterial wall of a control mouse ( P < 0.001). Laser ablation-inductively coupled plasma-mass spectrometry confirmed strong colocalization of areas positive for ADAMTS4 and Gd.
Conclusions: Magnetic resonance imaging using an ADAMTS4-specific agent is a promising method for characterizing atherosclerotic plaques and could improve plaque assessment in the diagnosis and treatment of atherosclerosis.
Keywords: ADAMTS4; atherosclerosis; inflammation; molecular imaging; mouse model; plaque; vulnerability.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Conflicts of interest and sources of funding: The authors declare that they have no competing interests. This work was supported by the Deutsche Forschungsgemeinschaft/German Research Foundation (Project-ID 372486779-SFB 1340, B01, B02).
Figures


References
-
- Virmani R Burke AP Farb A, et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–C18. - PubMed
-
- Cybulsky MI, Gimbrone MA. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. - PubMed
-
- Libby P Buring JE Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

