Hydrogen-Rich Saline Combined With Vacuum Sealing Drainage Promotes Wound Healing by Altering Biotin Metabolism
- PMID: 39804806
- PMCID: PMC11728484
- DOI: 10.1111/jcmm.70292
Hydrogen-Rich Saline Combined With Vacuum Sealing Drainage Promotes Wound Healing by Altering Biotin Metabolism
Abstract
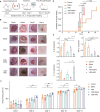
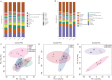
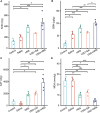
Impaired wound healing affects the life quality of patients and causes a substantial financial burden. Hydrogen-rich medium is reported to have antioxidant and anti-inflammatory effects. However, the role of hydrogen-rich saline (HRS) in cutaneous wound healing remains largely unexplored, especially by metabolomics. Thus, untargeted metabolomics profiling was analysed to study the effects and mechanism of HRS combined with vacuum sealing drainage (VSD) in a rabbit full-thickness wound model. Our results indicated that the combination treatment of HRS and VSD could accelerate wound healing. In vitro experiments further confirmed its effects on HaCaT keratinocytes. We found that 45 metabolites were significantly changed between the VSD + HRS group and the VSD + saline-treated group. Pathway enrichment analysis indicated that biotin metabolism was the potential target pathway. The biochemical interpretation analysis demonstrated that combining HRS and VSD might enhance mitochondrial function, ATP synthesis, and GSH homeostasis by altering biotin metabolism. The detection of representative indicators of oxidative stress supported the critical metabolic pathway analysis as well. In summary, VSD combined with HRS might provide a new strategy to enhance wound healing.
Keywords: biotin metabolism; hydrogen‐rich saline; metabolomics; oxidative stress; vacuum sealing drainage; wound healing.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Yildirimer L., Thanh N. T., and Seifalian A. M., “Skin Regeneration Scaffolds: A Multimodal Bottom‐Up Approach,” Trends in Biotechnology 30 (2012): 638–648. - PubMed
-
- Modaresifar K., Azizian S., Ganjian M., Fratila‐Apachitei L. E., and Zadpoor A. A., “Bactericidal Effects of Nanopatterns: A Systematic Review,” Acta Biomaterialia 83 (2019): 29–36. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

